 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,1-Benzoxazole | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.005.437 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NO | |
| Molar mass | 119.123 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.183 g/mL[1] |
| Boiling point | 101–102 °C (214–216 °F; 374–375 K)[1] |
| Hazards | |
| GHS labelling: | |
 [1] [1] | |
| Warning | |
| H302[1] | |
| P264, P270, P301+P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
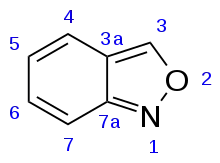
Anthranil (2,1-benzisoxazole) is an organic compound with a molecular formula C7H5NO, which features a fused benzene-isoxazole bicyclic ring structure. It is an isomer of the more common compounds benzoxazole and benzisoxazole, which have their oxygen atoms located in the 1-position. The locations of the heteroatoms in anthranil results in disrupted aromaticity, making it by far the least stable of the 3 structural isomers.[2]
References
- 1 2 3 4 Sigma-Aldrich Co., Anthranil. Retrieved on 2017-03-02.
- ↑ Domene, Carmen; Jenneskens, Leonardus W.; Fowler, Patrick W. (2005). "Aromaticity of anthranil and its isomers, 1,2-benzisoxazole and benzoxazole". Tetrahedron Letters. 46 (23): 4077–4080. doi:10.1016/j.tetlet.2005.04.014. hdl:1874/14837. ISSN 0040-4039.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.