 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
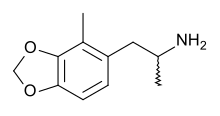
| Formula | C11H15NO2 |
| Molar mass | 193.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2-Methyl-3,4-methylenedioxyamphetamine (2-methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class. It acts as a selective serotonin releasing agent (SSRA), with IC50 values of 93nM, 12,000nM, and 1,937nM for serotonin, dopamine, and norepinephrine efflux.[1] 2-Methyl-MDA is more potent than MDA and 5-methyl-MDA.[1] However, it is slightly more selective for serotonin over dopamine and norepinephrine release in comparison to 5-methyl-MDA.[1]
References
- 1 2 3 Parker MA, Marona-Lewicka D, Kurrasch D, Shulgin AT, Nichols DE (March 1998). "Synthesis and pharmacological evaluation of ring-methylated derivatives of 3,4-(methylenedioxy)amphetamine (MDA)". Journal of Medicinal Chemistry. 41 (6): 1001–5. CiteSeerX 10.1.1.688.9559. doi:10.1021/jm9705925. PMID 9526575.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.