 | |||
| |||
| Names | |||
|---|---|---|---|
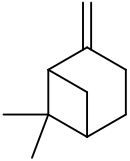
| IUPAC names
6,6-Dimethyl-2-methylidenebicyclo[3.1.1]heptane Pin-2(10)-ene | |||
| Other names
6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane 2(10)-Pinene Nopinene Pseudopinene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.004.430 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.872 g/mL | ||
| Melting point | −61.54 °C; −78.77 °F; 211.61 K[1] | ||
| Boiling point | 165–167 °C; 329–332 °F; 438–440 K[2] | ||
| Thermochemistry | |||
Std enthalpy of combustion (ΔcH⦵298) |
−6214.1±2.9 kJ/mol[1] | ||
| Hazards | |||
| GHS labelling: | |||
  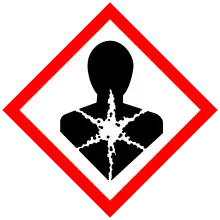 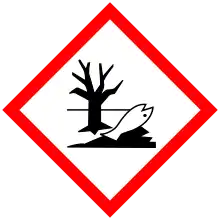 | |||
| Danger | |||
| H226, H304, H315, H317, H410 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P272, P273, P280, P301+P310, P302+P352, P303+P361+P353, P321, P331, P332+P313, P333+P313, P362, P363, P370+P378, P391, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 36 °C (97 °F; 309 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
β-Pinene is a monoterpene, an organic compound found in plants. It is one of the two isomers of pinene, the other being α-pinene. It is colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.
This is one of the most abundant compounds released by forest trees.[3] If oxidized in air, the allylic products of the pinocarveol and myrtenol family prevail.[4]
Sources
Many plants from many botanical families contain the compound, including:
Uses
β-Pinene is used in fragrances and essential oils. It is also used in the production of other aroma compounds, such as myrcene and nerol.
References
- 1 2 "β-Pinene". National Institute of Standards and Technology. Retrieved January 29, 2018.
- ↑ "(−)-β-Pinene". Sigma-Aldrich. Retrieved January 29, 2018.
- ↑ Geron, C., et al. (2000). A review and synthesis of monoterpene speciation from forests in the United States. Atmospheric Environment 34(11), 1761-81.
- 1 2 Neuenschwander, U.; Meier, E.; Hermans, I. (2011). "Peculiarities of β-pinene autoxidation". ChemSusChem. 4 (11): 1613–21. doi:10.1002/cssc.201100266. PMID 21901836.
- ↑ Li, Rong; Jiang, Zi-Tao (2004). "Chemical composition of the essential oil of Cuminum cyminum L. From China". Flavour and Fragrance Journal. 19 (4): 311–313. doi:10.1002/ffj.1302.
- ↑ Wang, L.; Wang, Z.; Zhang, H.; Li, X.; Zhang, H. (2009). "Ultrasonic nebulization extraction coupled with headspace single drop microextraction and gas chromatography-mass spectrometry for analysis of the essential oil in Cuminum cyminum L". Analytica Chimica Acta. 647 (1): 72–7. doi:10.1016/j.aca.2009.05.030. PMID 19576388.
- ↑ Tinseth, G. The Essential Oil of Hops: Hop Aroma and Flavor in Hops and Beer. Archived 2013-11-11 at the Wayback Machine Brewing Techniques January/February 1994. Accessed July 21, 2010.
- ↑ Hillig, Karl W (October 2004). "A chemotaxonomic analysis of terpenoid variation in Cannabis". Biochemical Systematics and Ecology. 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004. ISSN 0305-1978.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
-(-)-beta-pinene-2D-projected-skeletal.png.webp)
-(%E2%88%92)-beta-pinene-from-xtal-3D-balls.png.webp)
