| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Carbonyl difluoride | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.005.941 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2417 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| COF2 | |||
| Molar mass | 66.007 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Pungent and very irritating[1] | ||
| Density | 2.698 g/L (gas), 1.139 g/cm3 (liquid at melting point) | ||
| Melting point | −111.26 °C (−168.27 °F; 161.89 K) | ||
| Boiling point | −84.57 °C (−120.23 °F; 188.58 K) | ||
| Reacts[2] | |||
| Vapor pressure | 55.4 atm (20°C)[2] | ||
| Structure | |||
| C2v | |||
| 0.95 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Very toxic, reacts with water to release HF | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H280, H290, H314, H330, H331, H370 | |||
| P234, P260, P261, P264, P270, P271, P280, P284, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P310, P311, P320, P321, P363, P390, P403+P233, P404, P405, P410+P403, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[2] | ||
REL (Recommended) |
TWA 2 ppm (5 mg/m3) ST 5 ppm (15 mg/m3)[2] | ||
IDLH (Immediate danger) |
N.D.[2] | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
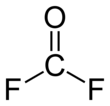
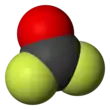
Carbonyl fluoride is a chemical compound with the formula COF2. It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with C2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°.[3]
Preparation and properties
Carbonyl fluoride is usually produced as a decomposition product of fluorinated hydrocarbons in the thermal decomposition thereof, for example from trifluoromethanol or tetrafluoromethane in the presence of water:
- CF4 + H2O → COF2 + 2 HF
Carbonyl fluoride can also be prepared by reaction of phosgene with hydrogen fluoride and the fluorination of carbon monoxide, although the latter tends to result in over-fluorination to carbon tetrafluoride. The fluorination of carbon monoxide with silver difluoride is convenient:
- CO + 2 AgF2 → COF2 + 2 AgF
Carbonyl fluoride is unstable in the presence of water, hydrolyzing to carbon dioxide and hydrogen fluoride:[4]
- COF2 + H2O → CO2 + 2 HF
Safety
Carbonyl fluoride is very toxic with a recommended exposure limit of 2 ppm as an 8-hour time weighted average and a 5 ppm as a short-term (15-minute average) exposure, where 1 ppm = 2.70 mg of carbonyl fluoride per 1 m3 of air.[1]
References
- 1 2 "Carbonyl Fluoride". NIOSH Pocket Guide to Chemical Hazards. CDC Centers for Disease Control and Prevention. Retrieved 2013-09-10.
- 1 2 3 4 5 NIOSH Pocket Guide to Chemical Hazards. "#0108". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 304–305. ISBN 978-0-08-037941-8.
- ↑ M. W. Farlow; E. H. Man; C. W. Tullock (1960). "Carbonyl Fluoride". Inorganic Syntheses. Inorganic Syntheses. Vol. 6. pp. 155–158. doi:10.1002/9780470132371.ch48. ISBN 9780470132371.