 | |
 | |
 | |
| Names | |
|---|---|
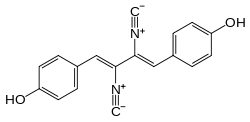
| Preferred IUPAC name
4,4′-[(1Z,3Z)-2,3-Diisocyanobuta-1,3-diene-1,4-diyl]diphenol | |
| Other names
Xanthocillin X, Ophthocillin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| Properties | |
| C18H12N2O2 | |
| Molar mass | 288.306 g·mol−1 |
| Appearance | Yellow crystals |
| Melting point | 200 °C (392 °F; 473 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Xantocillin[1] (INN), also known as xanthocillin X or ophthocillin, was the first reported natural product found to contain the isocyanide functional group. It was first isolated from Penicillium notatum by Rothe in 1950[2] and subsequently from several other sources.[3][4]
See also
References
- ↑ PubChem. "Xantocillin". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-11-03.
- ↑ W. ROTHE (1950). "Vorläufige Mitteilung über eine neues Antibiotikum". Pharmazie. 5: 190.
- ↑ Paul J. Scheuer (1992). "Isocyanides and cyanides as natural products". Accounts of Chemical Research. 25 (10): 433–439. doi:10.1021/ar00022a001.
- ↑ Kozlovskiĭ AG, Zhelifonova VP, Antipova TV, Adanin VM, Novikova ND, Deshevaia EA, et al. (2004). "[Penicillium expansum, a resident fungal strain of the orbital complex Mir, producing xanthocillin X and questiomycin A]". Prikl Biokhim Mikrobiol. 40 (3): 344–9. PMID 15283339.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.