Gamma-Hydroxybutyraldehyde
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Hydroxybutanal | |
| Other names
GHBAL, γ-hydroxybutaldehyde, γ-hydroxybutanal | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.042.900 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C4H8O2 |
| Molar mass | 88.106 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.109 g/cm3 (at 12 °C) |
| Boiling point | 65–68 °C (149–154 °F; 338–341 K) 10 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
γ-Hydroxybutyraldehyde is the organic compound with the formula HOCH2CH2CH2CHO. It is a colorless liquid. The compound occurs in nature and is produced commercially.[1]
Occurrence
It is a chemical intermediate in the biosynthesis of the neurotransmitter γ-hydroxybutyric acid (GHB) from 1,4-butanediol (1,4-BD).[2] Like 1,4-BD, it also behaves as a prodrug to GHB when taken exogenously. However, as with all aliphatic aldehydes, γ-hydroxybutaldehyde is caustic and is strong-smelling and foul-tasting; thus, actual ingestion of this compound is likely to be unpleasant and result in severe nausea and vomiting.
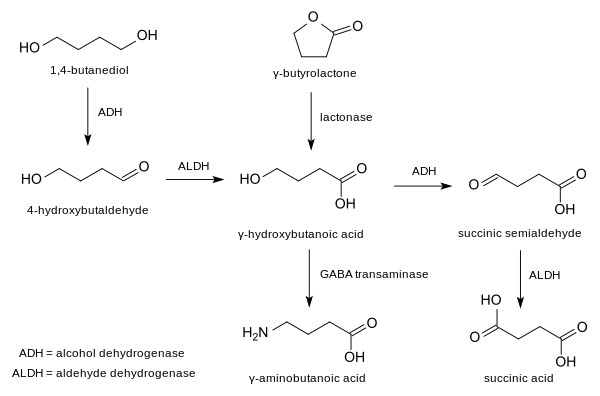
Metabolic pathway of GHB.
See also
- 1,6-Dioxecane-2,7-dione
- 3-Hydroxybutanal
- Aceburic acid
- Ethyl acetoxy butanoate
References
- ↑ Ahn, H. (1999). "Hydroformylation of olefins with formaldehyde in the presence of RhHCO(PPh3)3". Journal of Molecular Catalysis A: Chemical. 144 (2): 295–306. doi:10.1016/S1381-1169(99)00002-3.
- ↑ Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 413–. ISBN 978-1-60913-345-0.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.