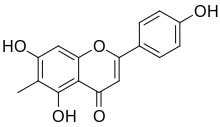
6-Methylapigenin
 | |
| Names | |
|---|---|
| IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-methylchromen-4-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C16H12O5 |
| Molar mass | 284.267 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
6-Methylapigenin is a naturally occurring flavonoid and a derivative of apigenin. It has activity at GABAA receptors as a positive modulator.
Natural occurrence
6-Methylapigenin can be found in multiple plants, such as Valeriana officinalis, Valeriana jatamansi, and Picea neoveitchii.[1]
Biological activity
6-Methylapigenin binds to the GABAA receptor on the benzodiazepine binding site. This compound possesses anxiolytic effects. In a mouse model, it is also able to potentiate sleep induced by hesperidin, another flavonoid.[2][3] However, since it does not have the chemical structure of benzodiazepines, it can therefore be classed as a nonbenzodiazepine.
References
- ↑ PubChem. "6-Methylapigenin". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-02-04.
- ↑ Fernández, Sebastián P.; Wasowski, Cristina; Paladini, Alejandro C.; Marder, Mariel (2005-04-11). "Synergistic interaction between hesperidin, a natural flavonoid, and diazepam". European Journal of Pharmacology. 512 (2–3): 189–198. doi:10.1016/j.ejphar.2005.02.039. ISSN 0014-2999. PMID 15840404.
- ↑ Marder, Mariel; Viola, Haydeé; Wasowski, Cristina; Fernández, Sebastián; Medina, Jorge H.; Paladini, Alejandro C. (2003). "6-methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS". Pharmacology, Biochemistry, and Behavior. 75 (3): 537–545. doi:10.1016/s0091-3057(03)00121-7. ISSN 0091-3057. PMID 12895671. S2CID 37559366.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.