BOHD (psychedelic)
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
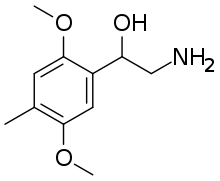
2-Amino-1-(2,5-dimethoxy-4-methylphenyl)ethan-1-ol | |
| Other names
4-Methyl-2,5-dimethoxy-beta-hydroxyphenethylamine 2-(4-Methyl-2,5-dimethoxyphenyl)ethan-beta-hydroxyamine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H17NO3 |
| Molar mass | 211.261 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
BOHD (4-methyl-2,5-dimethoxy-beta-hydroxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-hydroxy derivative of 2C-D. BOHD was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 50 mg, and the duration unknown.[1] BOHD produces a marked drop in blood pressure.[1] Very little data exists about the pharmacological properties, metabolism, and toxicity of BOHD.
Legality
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[2]
United States
In the U.S., this substance is a Schedule 1 isomer of Mescaline.
See also
References
- 1 2 Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ↑ "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Retrieved 12 March 2014.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.