Carbol fuchsin
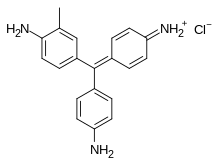 Chemical structure of fuchsin | |
| Names | |
|---|---|
| IUPAC name
4-[(E)-(4-aminophenyl)-(4-imino-3-methylcyclohexa-2,5-dien-1-ylidene)methyl]-2-methylaniline;hydrochloride | |
| Other names
Carbol-Fuchsin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.021.897 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C26H25N3O·HCl |
| Molar mass | 351.9 g/mol |
| Hazards | |
| GHS labelling:[1] | |
Pictograms |
  |
Signal word |
Warning |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Carbol fuchsin, carbol-fuchsin, carbolfuchsin, or Castellani's paint (CAS ) is a mixture of phenol and basic fuchsin that is used in bacterial staining procedures. It is commonly used in the staining of mycobacteria because it has an affinity for the mycolic acids found in their cell membranes.
It is a component of Ziehl–Neelsen stain, a differential stain.[2][3] Carbol fuchsin is used as the primary stain dye to detect acid-fast bacteria because it is more soluble in the cells' wall lipids than in the acid alcohol. If the bacteria is acid-fast the bacteria will retain the initial red color of the dye because they are able to resist the destaining by acid alcohol (0.4–1% HCl in 70% EtOH).[4] Additionally, it can be used for the staining of bacterial spores.
Carbol-fuchsin is also used as a topical antiseptic and antifungal.
References
- ↑ PubChem. "Carbol-Fuchsin". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-07-29.
- ↑ Angra P, Ridderhof J, Smithwick R (July 2003). "Comparison of two different strengths of carbol fuchsin in Ziehl-Neelsen staining for detecting acid-fast bacilli". J. Clin. Microbiol. 41 (7): 3459. doi:10.1128/JCM.41.7.3459.2003. PMC 165351. PMID 12843125.
- ↑ Selvakumar N, Rahman F, Rajasekaran S, et al. (August 2002). "Inefficiency of 0.3% carbol fuchsin in ziehl-neelsen staining for detecting acid-fast bacilli". J. Clin. Microbiol. 40 (8): 3041–3. doi:10.1128/JCM.40.8.3041-3043.2002. PMC 120628. PMID 12149374.
- ↑ Sokolovská, Ivana; Rozenberg, Raoul; Riez, Christophe; et al. (2003-12-01). "Carbon Source-Induced Modifications in the Mycolic Acid Content and Cell Wall Permeability of Rhodococcus erythropolis E1". Applied and Environmental Microbiology. 69 (12): 7019–7027. Bibcode:2003ApEnM..69.7019S. doi:10.1128/AEM.69.12.7019-7027.2003. ISSN 0099-2240. PMC 309960. PMID 14660344.