Cefluprenam
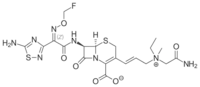 | |
| Names | |
|---|---|
| IUPAC name
(6R,7R)-3-[(E)-3-[(2-amino-2-oxoethyl)-ethyl-methylazaniumyl]prop-1-enyl]-7-[[(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(fluoromethoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate | |
| Other names
Antibiotic E 1077 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C20H25FN8O6S2 |
| Molar mass | 556.59 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cefluprenam is a fourth generation cephalosporin.[1] It was patented in 2008 by now defunct Cubist Pharmaceuticals.[2] A 1997 clinical trial illustrated that Cefluprenam is highly effective against bacterial pneumonia.[3]
References
- ↑ PubChem. "Cefluprenam". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-08-03.
- ↑ EU patent 2379580B1, Pearson, Lee Andre; Metcalf, A. Chester & Li, Jing, "Novel antibacterial agents for the treatment of gram positive infections", published 2011-10-26, issued 2013-10-23
- ↑ Shimada, K.; Ohmichi, M.; Sasaki, H.; Watanabe, A.; Sato, K.; Nagai, K.; Konishi, K.; Ohta, T.; Matsuura, Y.; Ikeda, H.; Igarashi, K. (August 1997). "[Usefulness of 7 day therapy with cefluprenam in the management of respiratory tract infections]". Kansenshogaku Zasshi. The Journal of the Japanese Association for Infectious Diseases. 71 (8): 770–787. doi:10.11150/kansenshogakuzasshi1970.71.770. ISSN 0387-5911. PMID 9311195.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.