Dupracetam
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.279 |
| Chemical and physical data | |
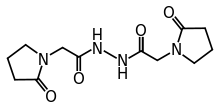
| Formula | C12H18N4O4 |
| Molar mass | 282.300 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Dupracetam is a nootropic drug from the racetam family.[1][2]
One of its metabolites, 1-Methylhydantoin, displays renal toxicity in high doses.[3]
See also
References
- ↑ Dell HD, Jacobi H, Kamp R, Kurz J, Wünsche C (August 1981). "[1-Methylhydantoin, an unexpected metabolite of the intelligence-affecting substance dupracetam (author's transl)]". Archiv der Pharmazie (in German). 314 (8): 697–702. doi:10.1002/ardp.19813140808. PMID 7294979. S2CID 95603731.
- ↑ Hall ED, Von Voigtlander PF (November 1987). "Facilitatory effects of piracetam on excitability of motor nerve terminals and neuromuscular transmission". Neuropharmacology. 26 (11): 1573–1579. doi:10.1016/0028-3908(87)90003-7. PMID 2829047. S2CID 7759558.
- ↑ Yang B, Liu D, Li CZ, Liu FY, Peng YM, Jiang YS (2007). "1-Methylhydantoin cytotoxicity on renal proximal tubular cells in vitro". Renal Failure. 29 (8): 1025–1029. doi:10.1080/08860220701641272. PMID 18067051. S2CID 26843776.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.