GC376
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H30N3NaO8S |
| Molar mass | 507.53 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
GC376 is a broad-spectrum antiviral medication under development by the biopharmaceutical company Anivive Lifesciences for therapeutic uses in humans and animals.[1] Anivive licensed the exclusive worldwide patent rights to GC376 from Kansas State University.[2] As of 2020, GC376 is being investigated as treatment for COVID-19.[3] GC376 shows activity against many human and animal viruses including coronavirus and norovirus;[4] the most extensive research has been multiple in vivo studies in cats treating a coronavirus which causes deadly feline infectious peritonitis.[5] Other research supports use in porcine epidemic diarrhea virus.[6]
COVID-19
Since GC376 shows broad-spectrum activity against coronavirus,[7] early on during the pandemic of 2020 it was suggested as a potential treatment for COVID-19.[8] In response to the crisis, researchers at the University of Arizona published in vitro research indicating GC376 is highly active against 3CLpro in SARS-CoV-2 (the coronavirus which causes COVID-19).[9] Another group of virologists at the University of Alberta led by D. Lorne Tyrrell then released a separate publication confirming GC376's activity against 3CLpro in SARS-CoV-2 and also indicating GC376 had a potent antiviral effect.
Pharmacology
Pharmacodynamics
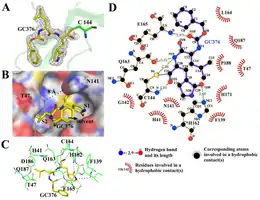
GC376 is a protease inhibitor. It blocks the 3CLpro, a protease common to many (+)ssRNA viruses, thereby preventing the viral polyprotein from maturing into its functional parts. Chemically, GC376 is the bisulfite adduct of an aldehyde GC373 and it behaves as a prodrug for that compound. This aldehyde forms a covalent bond with the cysteine-144 residue at the protease's active site, giving a monothioacetal and blocking the enzyme's normal function.[6][10]
See also
- 3CLpro-1
- Carmofur
- Ebselen
- GRL-0617
- Rupintrivir
- Theaflavin digallate
- PF-07321332 (Pfizer)
References
- ↑ "Anivive". www.anivive.com. Retrieved 2020-05-14.
- ↑ "Anivive licenses antiviral drug for fatal cat disease". www.k-state.edu. September 20, 2018. Retrieved 2020-05-14.
- ↑ Stockwell B (6 May 2020). "COVID-19 Virtual Symposium". Retrieved 2020-05-14 – via YouTube.
- ↑ Takahashi D, Kim Y, Lovell S, Prakash O, Groutas WC, Chang KO (December 2013). "Structural and inhibitor studies of norovirus 3C-like proteases". Virus Research. 178 (2): 437–444. doi:10.1016/j.virusres.2013.09.008. PMC 3840063. PMID 24055466.
- ↑ Pedersen NC, Kim Y, Liu H, Galasiti Kankanamalage AC, Eckstrand C, Groutas WC, et al. (April 2018). "Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis". Journal of Feline Medicine and Surgery. 20 (4): 378–392. doi:10.1177/1098612X17729626. PMC 5871025. PMID 28901812.
- 1 2 3 Ye G, Wang X, Tong X, Shi Y, Fu ZF, Peng G (February 2020). "Structural Basis for Inhibiting Porcine Epidemic Diarrhea Virus Replication with the 3C-Like Protease Inhibitor GC376". Viruses. 12 (2): 240. doi:10.3390/v12020240. PMC 7077318. PMID 32098094.
- ↑ Pillaiyar T, Meenakshisundaram S, Manickam M (April 2020). "Recent discovery and development of inhibitors targeting coronaviruses". Drug Discovery Today. 25 (4): 668–688. doi:10.1016/j.drudis.2020.01.015. PMC 7102522. PMID 32006468.
- ↑ Morse JS, Lalonde T, Xu S, Liu WR (March 2020). "Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV". ChemBioChem. 21 (5): 730–738. doi:10.1002/cbic.202000047. PMC 7162020. PMID 32022370.
- ↑ Ma C, Sacco MD, Hurst B, Townsend JA, Hu Y, Szeto T, et al. (January 2020). "Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease". bioRxiv. doi:10.1101/2020.04.20.051581. PMC 7263507. PMID 32511378. S2CID 216145410.
- ↑ Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, et al. (August 2020). "Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication". Nature Communications. 11 (1): 4282. doi:10.1038/s41467-020-18096-2. PMC 7453019. PMID 32855413. S2CID 218551888.