Thiomargarita namibiensis
| Thiomargarita namibiensis | |
|---|---|
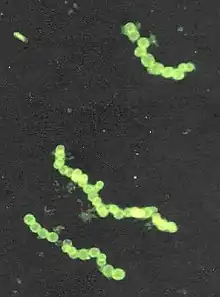 | |
| Stained micrograph of Thiomargarita namibiensis | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Thiotrichales |
| Family: | Thiotrichaceae |
| Genus: | Thiomargarita |
| Species: | T. namibiensis |
| Binomial name | |
| Thiomargarita namibiensis Schulz et al., 1999 | |
Thiomargarita namibiensis is a harmless, gram-negative, facultative anaerobic, coccoid bacterium found in the ocean sediments of the continental shelf of Namibia. [1] It is the second largest bacterium ever discovered, at 0.1–0.3 mm (100–300 μm) in diameter on average, but can attain up to 0.75 mm (750 μm).[2][3] Cells of Thiomargarita namibiensis are large enough to be visible to the naked eye. The previously largest known bacterium was Epulopiscium fishelsoni, at 0.5mm long.[4] The current largest known bacterium is Thiomargarita magnifica, described in 2022, at an average length of 10 mm. [5] [6]
This baacterium is categorized as a mesophile because of its preference for moderate temperatures, which typically range between 20-45 degrees Celsius. The organism shows neutrophilic characteristics by favoring environments with neutral pH levels like 6.5-7.5. This highlights the bacterium's unique strategies to maintain its survival and grow. [7]
The genus name Thiomargarita means "sulfur pearl." This refers to the appearance of the cells; they contain microscopic sulfur granules that scatter incident light, lending the cell a pearly luster. This causes the cells to form chains, resembling strings of pearls. The species name namibiensis means "of Namibia", which is an ode to their country of discovery and existence. Together, Thiomargarita namibiensis means “Sulfur pearl of Namibia".[8]
Discovery
The species Thiomargarita namibiensis was collected in 1997 and discovered in 1999 by Heide N. Schulz and her colleagues from the Max Planck Institute for Marine Microbiology. It was discovered in coastal sediments on the Namibian coast of West Africa. Schulz and her colleagues were aboard the Russian research vessel Petr Kottsov in search of Thioploca, another recently discovered sulfide-eating marine bacteria. Schulz's team found small quantities of Thioploca and Beggiatoa in sediment samples, but large quantities of the previously undiscovered Thiomargarita namibiensis.[9] Researchers suggested the species be named Thiomargarita namibiensis, which means "sulfur pearl of Namibia". [10]
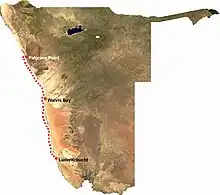
The Namibian coastal environmental experiences strong upwelling, resulting in low oxygen levels in the lower waters with large amounts of plankton. Thiomargarita namibiensis is most prevalent in the Walvis Bay area at 300 feet deep, [11] but they are distributed along the coast of Namibia from Palgrave Point to Lüderitzbucht.[12] The highest number of Thiomargarita namibiensis is present in the sediment's uppermost three centimeters; they form a film on top of the sediment, detoxifying the sulfide so that it is able to enter the water column.[13] Since the Thiomargarita namibiensis are immobile, they are unable to seek more a more ideal environment when sulfide and nitrate levels are low in this environment. They simply remain in position and wait for levels to increase once again so that they can undergo respiration and other processes. [14]
In 2002 a strain exhibiting 99% identity with Thiomargarita namibiensis was found in sediment cores taken from the Gulf of Mexico during a research expedition. This similar strain either occurs in single cells or clusters of 2, 4, and 8 cells, as opposed to the Namibian strain which occurs in single chains of cells separated by a thin mucus sheath.[15]
Occurrence
Thiomargarita namibiensis was found in the continental shelf off the coast of Namibia, an area with high plankton productivity, low oxygen concentrations between 0-3 μM, and nitrate concentrations of 5-28 μM.[9] The most bacteria were obtained from the upper 3 cm of sediment in the sample, with concentrations decreasing exponentially past this point. In this section of sediment, there were sulfide concentrations of 100-800 μM.[9] Although previously undiscovered, T. namibiensis is not uncommon by any means in its environment. It is by far the most common benthos bacterium of the Namibian shelf, comprising almost 0.8% of the sediment volume.[16] The organism also has a direct impact on its environment. Apatite, a mineral high in phosphorite, is correlated with the abundance of T. namibiensis through phosphogenesis. Internal polyphosphate and nitrate are used as external electron acceptors in the presence of acetate, releasing enough phosphate to cause precipitation. While the amount directly created by T. namibiensis cannot be calculated, it is a significant contribution to the large amounts of hydroxyapatite in solid-phase shelf sediment.[17]
The Mexican strain was primarily found in the top centimeter of sediment sampled from cold seeps in the Gulf of Mexico. The top 3 cm of sediment from the Gulf of Mexico locations contained sulfide concentrations of 200-1900 μM.[15]
Structure
Although Thiomargarita are closely related to Thioploca and Beggiatoa in function, their structures are different. Thioploca and Beggiatoa cells are much smaller and grow tightly stacked on each other in long filaments. Their shape is necessary for them to shuttle down into the ocean sediments to find more sulfide and nitrate. In contrast, Thiomargarita grow in rows of separate single ball-shaped cells, so they lack the range of mobility that Thioploca and Beggiota have. Thiomargarita can also grow in barrel shapes. The spherical shaped Thiomargarita can join together to create chains of 4-20 cells, while the barrel shaped Thiomargarita can form chains of more than 50 cells. These chains are not linked together by filaments, but connected by a mucus sheath.[18]
With their lack of movement, Thiomargarita have adapted by evolving very large nitrate-storing bubbles, called vacuoles, allowing them to survive long periods of nitrate and sulfide starvation. The vacuoles give them the ability to stay immobile, just waiting for nitrate-rich waters to sweep over them once again. These vacuoles are what account for the size that scientists had previously thought impossible, and account for roughly 98% of the cell volume.[19] Scientists disregarded large bacteria, because bacteria rely on chemiosmosis and cellular transport processes across their membranes to make ATP. As the cell size increases, they make proportionately less ATP, thus energy production limits their size. Thiomargarita are an exception to this size constraint, as their cytoplasm forms along the periphery of the cell, while the nitrate-storing vacuoles occupy the center of the cell. As these vacuoles swell, they greatly contribute to the record sizes of Thiomargarita cells. T. namibiensis holds the record for the world's second largest bacteria, with a volume three million times more than that of the average bacteria.[20]
Being that areas of nitrate and hydrogen sulfide do not mix together and T. namibiensis cells are immobile, the storage vacuoles in the cell provide a solution to this problem. Because of these storage vacuoles, cells are able to stay viable without growing (or dividing), with low relative amounts of cellular protein, and large amounts of nitrogen in the vacuoles. The storage vacuoles provide a novel solution which allows cells to wait for changing conditions while staying alive.[9]
Thiomargarita namibiensis have large vacuoles for their chemolithotrophic metabolism. The vacuoles of Thiomargarita namibiensis contribute to their gigantism, allowing them to store nutrients for asexual reproduction of their complex genome.
Metabolism
The bacterium is chemolithotrophic and is capable of using nitrate as the terminal electron acceptor in the electron transport chain. The organism will oxidize hydrogen sulfide (H2S) into elemental sulfur (S). This is deposited as granules in its periplasm and is highly refractile and opalescent, making the organism look like a pearl.
The large vacuole mainly stores nitrate for sulfur oxidation, the main energy source for T. namibiensis.[21] Large amounts of nitrogen must be stored as a terminal electron acceptor in the electron transport chain. Because of this and the organism's size, large amounts of sulfur are required which are then stored as cyclooctasulfur. The large amount of nitrogen helps T. namibiensis produce large amounts of energy, something that is necessary with the large size of the organism.
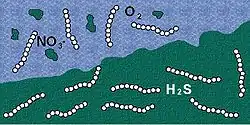
While the sulfide is available in the surrounding sediment, produced by other bacteria from dead microalgae that sank down to the sea bottom, the nitrate comes from the above seawater. Since the bacterium is sessile, and the concentration of available nitrate fluctuates considerably over time, it stores nitrate at high concentration (up to 0.8 molar[9]) in a large vacuole like an inflated balloon, which is responsible for about 80% of its size.[15] When nitrate concentrations in the environment are low, the bacterium uses the contents of its vacuole for respiration. T. namibiensis cells possess elevated nitrate concentrations making them able to exhibit the capacity to absorb oxygen both when nitrate is present and when it is not. Thus, the presence of a central vacuole in its cells enables a prolonged survival in sulfidic sediments. The non-motility of Thiomargarita cells is compensated by its large cellular size.[18] This immobility suggests that they rely on shifting chemical conditions.[22]
Cyclooctasulfur is stored in the globules of sulfur in the vacoules of T. namibiensis, aiding in their metabolism.[23] After the oxidation of sulfide, T. namibiensis stores sulfur as cyclooctasulfur, the most thermodynamically stable form of sulfur at standard temperature and pressure. With these sulfur globules in the cell, the organism uses it as storage of elemental sulfur in usually anoxic conditions to reduce the toxicity of various sulfur compounds (can also survive in atmospheric oxygen conditions as it is not toxic). The sulfur globules are stored in the thin outer layer of the cytoplasm, presumably after their use as a source of electrons in the electron transport chain through oxidation of sulfide.[23] The ability to oxidize hydrogen sulfide provides nutrients to other organisms living near it. [24]
The bacterium may be facultatively anaerobic rather than obligately anaerobic, and thus capable of respiring with oxygen if it is plentiful.[25]
Reproduction
T. namibiensis reproduces mainly through binary fission. Despite this, its cells remain connected, forming chains within a common mucus matrix. In addition to helping with essential functions including food exchange and cell-to-cell communication, this matrix can give the bacteria protection and structural support.[21] During the process of binary fission, a single bacterial cell divides into two identical daughter cells, representing a comparatively basic form of asexual reproduction. The cells that make up the filamentous chain may then separate into smaller segments, and each of those segments may go on to produce a new filament.[26]
Genome
The genome of this organism is complex. Instead of being distributed in a nucleoid region within the cytoplasm, T. namibiensis cells have their DNA concentrated in small masses around the inner side of the cell membrane. This could be used to regulate the processes of these large bacteria, as diffusion of protein and other material would take a long time in these large cells. This way of constructing its genome is very similar to the bacteria "Epulopiscium".[18]
Significance
Gigantism is usually a disadvantage for bacteria.[27] Bacteria obtain their nutrients via diffusion and cellular transport processes across their cell membrane, as they lack the sophisticated nutrient uptake mechanisms such as endocytosis found in eukaryotes. A bacterium of large size would imply a lower ratio of cell membrane surface area to cell volume. This would limit the rate of uptake of nutrients to threshold levels.[28] Large bacteria might starve easily unless they have a different backup mechanism.[29] T. namibiensis overcomes this problem by harboring large vacuoles that can be filled up with life-supporting nitrates.[30]
T. namibiensis plays a vital role in the sulfur and nitrogen cycles. In their sulfur rich environment, oxygen is scarcely available and cannot be used as an electron acceptor. In turn, T. namibiensis uses nitrate as the electron acceptor, which they consume at the sediment surface and condense in a vacuole. From this, they can oxidize the sulfide that inhabits the sediment. This acts as a detoxifier which removes poisonous gas from the water. This keeps the environment affable for fish and other marine living beings. This bacteria also plays an essential role in the phosphorus cycle of the sediment. T. namibiensis can release phosphate in anoxic sediments that possess high rates which contribute to the spontaneous precipitation of phosphorus-containing material. This plays an important role in the removal of phosphorus in the biosphere. [31]
See also
- Valonia ventricosa - a large, 5 centimetre-wide unicellular species of algae
- Thiomargarita magnifica - the current largest bacterium in the world, closely related to this organism
References
- ↑ "Giant Sulfur Bacteria Discovered off African Coast".
- ↑ "The largest Bacterium: Scientist discovers new bacterial life form off the African coast". Max Planck Institute for Marine Microbiology. 8 April 1999. Archived from the original on 20 January 2010.
- ↑ "Genus Thiomargarita". List of Prokaryotic names with Standing in Nomenclature.
- ↑ Randerson J (8 June 2002). "Record Breaker". New Scientist.
- ↑ "Record bacterium discovered as long as human eyelash". BBC News. 23 June 2022. Retrieved 23 June 2022.
- ↑ Devlin, Hannah (23 June 2022). "Scientists discover world's largest bacterium, the size of an eyelash". The Guardian.
- ↑ Schulz, H. N.; Jorgensen, B. B. (2001). "Big bacteria". Annual Review of Microbiology. 55: 105–137. doi:10.1146/annurev.micro.55.1.105. ISSN 0066-4227. PMID 11544351.
- ↑ "Giant Sulfur Bacteria Discovered off African Coast".
- 1 2 3 4 5 Schulz HN, Brinkhoff T, Ferdelman TG, Mariné MH, Teske A, Jorgensen BB (April 1999). "Dense populations of a giant sulfur bacterium in Namibian shelf sediments". Science. 284 (5413): 493–495. Bibcode:1999Sci...284..493S. doi:10.1126/science.284.5413.493. PMID 10205058. S2CID 32571118.
- ↑ "Giant Sulfur Bacteria Discovered off African Coast". whoi.edu.
- ↑ "Giant Sulfur Bacteria Discovered off African Coast". whoi.edu.
- ↑ "Distribution of Thiomargarita namibiensis along the namibian coast". 29 October 2007.
- ↑ Winkel, Salman-Carvalho, Woyke, Richter, Schulz-Vogt, Bailey Mubmann, Matthias, Verena, Tanja, Michael, Heide, Beverly, Jake, Marc (2016). "Single-cell Sequencing of Thiomargarita Reveals Genomic Flexibility for Adaptation to Dynamic Redox Conditions". Frontiers in Microbiology. 7. PubMed Central: 964. doi:10.3389/fmicb.2016.00964. PMC 4914600. PMID 27446006.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Giant Sulfur Bacteria Discovered off African Coast". whoi.edu.
- 1 2 3 Kalanetra KM, Joye SB, Sunseri NR, Nelson DC (September 2005). "Novel vacuolate sulfur bacteria from the Gulf of Mexico reproduce by reductive division in three dimensions". Environmental Microbiology. 7 (9): 1451–1460. Bibcode:2005EnvMi...7.1451K. doi:10.1111/j.1462-2920.2005.00832.x. PMID 16104867.
- ↑ Schulz, Heide (March 2002). "Thiomargarita namibiensis: Giant microbe holding its breath". ResearchGate. Retrieved 15 November 2023.
- ↑ Schulz, Heide N.; Schulz, Horst D. (21 January 2005). "Large Sulfur Bacteria and the Formation of Phosphorite". Science. 307 (5708): 416–418. Bibcode:2005Sci...307..416S. doi:10.1126/science.1103096. ISSN 0036-8075. PMID 15662012.
- 1 2 3 Schulz HN (2006). "The Genus Thiomargarita". The Prokaryotes. pp. 1156–1163. doi:10.1007/0-387-30746-X_47. ISBN 978-0-387-25496-8.
- ↑ Mendell, Jennifer E.; Clements, Kendall D.; Choat, J. Howard; Angert, Esther R. (6 May 2008). "Extreme polyploidy in a large bacterium". Proceedings of the National Academy of Sciences of the United States of America. 105 (18): 6730–6734. doi:10.1073/pnas.0707522105. ISSN 0027-8424. PMC 2373351. PMID 18445653.
- ↑ "The World's Largest Bacteria". Woods Hole Oceanographic Institution. October 2001. Archived from the original on 4 March 2016.
- 1 2 Levin PA, Angert ER (June 2015). "Small but Mighty: Cell Size and Bacteria". Cold Spring Harbor Perspectives in Biology. 7 (7): a019216. doi:10.1101/cshperspect.a019216. PMC 4484965. PMID 26054743.
- ↑ Winkel M, Salman-Carvalho V, Woyke T, Richter M, Schulz-Vogt HN, Flood BE, et al. (21 June 2016). "Single-cell Sequencing of Thiomargarita Reveals Genomic Flexibility for Adaptation to Dynamic Redox Conditions". Frontiers in Microbiology. 7: 964. doi:10.3389/fmicb.2016.00964. PMC 4914600. PMID 27446006.
- 1 2 Prange, Alexander; Chauvistré, Reinhold; Modrow, Hartwig; Hormes, Josef; Trüper, Hans G; Dahl, Christiane (2002). "Quantitative speciation of sulfur in bacterial sulfur globules: X-ray absorption spectroscopy reveals at least three different species of sulfur". Microbiology. 148 (1): 267–276. doi:10.1099/00221287-148-1-267. ISSN 1465-2080. PMID 11782519.
- ↑ Tabashsum, Zajeba; Alvarado-Martinez, Zabdiel; Houser, Ashley; Padilla, Joselyn; Shah, Nishi; Young, Alana (2020), Biswas, Debabrata; Rahaman, Shaik O. (eds.), "Contribution of Human and Animal to the Microbial World and Ecological Balance", Gut Microbiome and Its Impact on Health and Diseases, Cham: Springer International Publishing, pp. 1–18, doi:10.1007/978-3-030-47384-6_1, ISBN 978-3-030-47384-6, retrieved 10 February 2024
- ↑ Schulz HN, De Beer D (November 2002). "Uptake rates of oxygen and sulfide measured with individual Thiomargarita namibiensis cells by using microelectrodes". Applied and Environmental Microbiology. 68 (11): 5746–5749. Bibcode:2002ApEnM..68.5746S. CiteSeerX 10.1.1.335.4467. doi:10.1128/AEM.68.11.5746-5749.2002. PMC 129903. PMID 12406774.
- ↑ Shih, Yu-Ling; Rothfield, Lawrence (September 2006). "The Bacterial Cytoskeleton". Microbiology and Molecular Biology Reviews. 70 (3): 729–754. doi:10.1128/MMBR.00017-06. ISSN 1092-2172. PMC 1594594. PMID 16959967.
- ↑ Ledford H (8 May 2008). "Giant bacterium carries thousands of genomes". Nature News. doi:10.1038/news.2008.806.
- ↑ Mendell JE, Clements KD, Choat JH, Angert ER (May 2008). "Extreme polyploidy in a large bacterium". Proceedings of the National Academy of Sciences of the United States of America. 105 (18): 6730–4. doi:10.1073/pnas.0707522105. PMC 2373351. PMID 18445653.
- ↑ Schulz HN, Jorgensen BB (October 2001). "Big bacteria". Annual Review of Microbiology. 55 (1): 105–137. doi:10.1146/annurev.micro.55.1.105. PMID 11544351.
- ↑ "The World's Largest Bacteria". Woods Hole Oceanographic Institution. Retrieved 5 January 2016.
- ↑ Schulz, Heide N. (2006), "The Genus Thiomargarita", The Prokaryotes, New York, NY: Springer New York, pp. 1156–1163, doi:10.1007/0-387-30746-x_47, ISBN 978-0-387-25496-8, retrieved 4 April 2024