Vilanterol
 | |
| Names | |
|---|---|
| Trade names | With fluticasone: Breo Ellipta, Relvar Ellipta With umeclidinium: Anoro Ellipta With both: Trelegy Ellipta |
IUPAC name
| |
| Clinical data | |
| Main uses | COPD, asthma[1][2] |
| Side effects | Nose and throat irritation, thrush, chest pain[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By inhalation |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
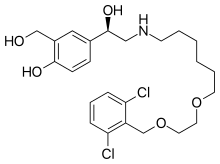
| Formula | C24H33Cl2NO5 |
| Molar mass | 486.43 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vilanterol is a medication primarily used to treat chronic obstructive pulmonary disease (COPD).[2] It may also be used to prevent worsening in asthma.[1] It is inhaled in combination with other medications.[1]
Common side effects include nose and throat irritation, thrush, and chest pain.[2] Other side effects may include anaphylaxis and bronchospasm.[2] It is a long-acting β2 adrenoreceptor agonist (LABA).[2]
Vilanterol in combination with fluticasone was approved for medical use in the United States in 2013.[3] It is also avaliable as umeclidinium/vilanterol and fluticasone/umeclidinium/vilanterol.[2][1] In the United Kingdom a month of medication costs the NHS about £20 to £45 as of 2021.[1] In the United States this amount costs about 190 USD.[4]
Medical uses
Combinations
Vilanterol is available in following combinations:
- with inhaled corticosteroid fluticasone furoate—fluticasone furoate/vilanterol (trade names Breo Ellipta (U.S.), Relvar Ellipta (EU, RU, JPN))
- with muscarinic antagonist umeclidinium bromide—umeclidinium bromide/vilanterol (trade name Anoro Ellipta)
- with inhaled corticosteroid fluticasone furoate and muscarinic antagonist umeclidinium bromide—fluticasone furoate/umeclidinium bromide/vilanterol (trade name Trelegy Ellipta)
Dosage
It is used by inhalation once per day.[1]
See also
- Salmeterol—a long-acting β2 adrenoreceptor agonist (LABA) with a similar backbone.
References
- 1 2 3 4 5 6 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 279. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 3 4 5 6 7 "Vilanterol Monograph for Professionals". Drugs.com. Archived from the original on 17 January 2021. Retrieved 15 September 2021.
- ↑ "FDA approves Breo Ellipta to treat chronic obstructive pulmonary disease". web.archive.org. 18 January 2017. Archived from the original on 18 January 2017. Retrieved 15 September 2021.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ↑ "Breo Ellipta Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 25 April 2020. Retrieved 15 September 2021.
External links
| Identifiers: |
|
|---|