 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.026.106 |
| Chemical and physical data | |
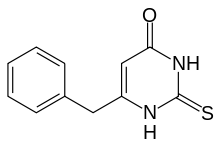
| Formula | C11H10N2OS |
| Molar mass | 218.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Benzylthiouracil (BTU) is an antithyroid preparation. It is a thioamide, closely related to propylthiouracil.
Adverse effects
Benzylthiouracil has been associated with severe adverse effects, notably vasculitis and subsequent ANCA-positive glomerulonephritis, as well as isolated reports of lung damage.[1][2][3][4]
References
- ↑ Tieulie N, Huong DL, Andreu M, et al. (2002). "[ANCA associated glomerulonephritis related to benzylthiouracil]". Rev Méd Interne (in French). 23 (10): 853–6. doi:10.1016/S0248-8663(02)00694-X. PMID 12428489.
- ↑ Kaaroud H, Khiari K, Ben Moussa F, Barbouch S, Boussema E, Ben Maïz H (2002). "[Vasculitis with renal and pulmonary involvement in a patient receiving benzylthiouracil for Graves disease]". Rev Méd Interne (in French). 23 (10): 857–61. doi:10.1016/S0248-8663(02)00704-X. PMID 12428490.
- ↑ Braham A, Houman MH, Rais L, Ben Gborbel I, Lamloum M, Miled M (2004). "[Benzylthiouracil induced ANCA-positive vasculitis]". Presse Médicale (in French). 33 (19 Pt 1): 1331–3. doi:10.1016/S0755-4982(04)98919-1. PMID 15615240.
- ↑ Thabet F, Sghiri R, Tabarki B, Ghedira I, Yacoub M, Essoussi AS (2006). "ANCA-associated diffuse alveolar hemorrhage due to benzylthiouracil". Eur J Pediatr. 165 (7): 435–6. doi:10.1007/s00431-005-0053-4. PMID 16622664.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.