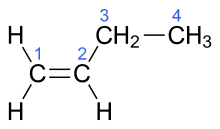 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
But-1-ene[1] | |||
| Other names
Ethylethylene 1-Butylene α-Butylene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1098262 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.137 | ||
| EC Number |
| ||
| 25205 | |||
PubChem CID |
|||
| UNII | |||
| UN number | 1012 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H8 | |||
| Molar mass | 56.108 g·mol−1 | ||
| Appearance | Colorless Gas | ||
| Odor | slightly aromatic | ||
| Density | 0.62 g/cm3 | ||
| Melting point | −185.3 °C (−301.5 °F; 87.8 K) | ||
| Boiling point | −6.47 °C (20.35 °F; 266.68 K) | ||
| 0.221 g/100 mL | |||
| Solubility | soluble in alcohol, ether, benzene | ||
Refractive index (nD) |
1.3962 | ||
| Viscosity | 7.76 Pa | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H220, H221, H280 | |||
| P210, P377, P381, P403, P410+P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −79 °C; −110 °F; 194 K | ||
| 385 °C (725 °F; 658 K) | |||
| Explosive limits | 1.6-10% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
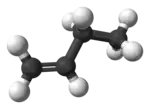
But-1-ene (or 1-butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin (terminal alkene).[2] It is one of the isomers of butene (butylene). It is a precursor to diverse products.
Reactions
Polymerization of but-1-ene gives polybutylene, which is used to make piping for domestic plumbing.[3] Another application is as a comonomer in the production of certain kinds of polyethylene, such as linear low-density polyethylene (LLDPE).[4] It has also been used as a precursor to polypropylene resins, butylene oxide, and butanone.[5]
Manufacturing
But-1-ene is produced by separation from crude C4 refinery streams and by ethylene dimerization. The former affords a mixture of 1-and 2-butenes, while the latter affords only the terminal alkene.[6] It is distilled to give a very high purity product. An estimated 12 billion kilograms were produced in 2011.[7]
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 17, 61, 374. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ "1-BUTENE". chemicalland21.com. Retrieved 22 April 2018.
- ↑ Whiteley, Kenneth S.; Heggs, T. Geoffrey; Koch, Hartmut; Mawer, Ralph L.; Immel, Wolfgang (2000). "Polyolefins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_487. ISBN 978-3527306732.
- ↑ Chum, P. Steve; Swogger, Kurt W. (2008). "Olefin polymer technologies—History and Recent Progress at the Dow Chemical Company". Progress in Polymer Science. 33 (8): 797–819. doi:10.1016/j.progpolymsci.2008.05.003.
- ↑ "1-Butene product overview". shell.com. Archived from the original on 2012-02-10. Retrieved 22 April 2018.
- ↑ "Alphabutol process - Big Chemical Encyclopedia". chempedia.info. Archived from the original on 2017-12-08. Retrieved 22 April 2018.
- ↑ Geilen, Frank M.A.; Stochniol, Guido; Peitz, Stephan; Schulte-Koerne, Ekkehard (2014). "Butenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_483.pub3. ISBN 978-3527306732.