 | |
 | |
| Names | |
|---|---|
| IUPAC name
Cesium bromide | |
| Other names
Cesium bromide, Caesium(I) bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.209 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CsBr | |
| Molar mass | 212.809 g/mol[1] |
| Appearance | White solid |
| Density | 4.43 g/cm3[1] |
| Melting point | 636 °C (1,177 °F; 909 K)[1] |
| Boiling point | 1,300 °C (2,370 °F; 1,570 K)[1] |
| 1230 g/L (25 °C)[1] Disputed.
420 g/L (11 °C) See References | |
| -67.2·10−6 cm3/mol[2] | |
Refractive index (nD) |
1.8047 (0.3 μm) 1.6974 (0.59 μm) 1.6861 (0.75 μm) 1.6784 (1 μm) 1.6678 (5 μm) 1.6439 (20 μm)[3] |
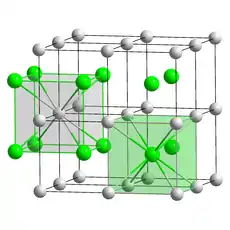
| Structure | |
| CsCl, cP2 | |
| Pm3m, No. 221[4] | |
a = 0.4291 nm | |
Lattice volume (V) |
0.0790 nm3 |
Formula units (Z) |
1 |
| Cubic (Cs+) Cubic (Br−) | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1400 mg/kg (oral, rat)[5] |
| Related compounds | |
Other anions |
Caesium fluoride Caesium chloride Caesium iodide Caesium astatide |
Other cations |
Sodium bromide Potassium bromide Rubidium bromide Francium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr. It is a white or transparent solid with melting point at 636 °C that readily dissolves in water. Its bulk crystals have the cubic CsCl structure, but the structure changes to the rocksalt type in nanometer-thin film grown on mica, LiF, KBr or NaCl substrates.[6]
Synthesis
Caesium bromide can be prepared via following reactions:
- CsOH (aq) + HBr (aq) → CsBr (aq) + H2O (l)
- Cs2(CO3) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H2O (l) + CO2 (g)
- Direct synthesis:
- 2 Cs (s) + Br2 (g) → 2 CsBr (s)
The direct synthesis is a vigorous reaction of caesium with bromine. Due to its high cost, it is not used for preparation.
Uses
Caesium bromide is sometimes used in optics as a beamsplitter component in wide-band spectrophotometers.
References
- 1 2 3 4 5 Haynes, p. 4.57
- ↑ Haynes, p. 4.132
- ↑ Haynes, p. 10.240
- ↑ Vallin, J.; Beckman, O.; Salama, K. (1964). "Elastic Constants of CsBr and CsI from 4.2K to Room Temperature". Journal of Applied Physics. 35 (4): 1222. Bibcode:1964JAP....35.1222V. doi:10.1063/1.1713597.
- ↑ Caesium bromide. nlm.nih.gov
- ↑ Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. doi:10.1107/S0365110X51001641.
* Crystran Ltd experimental data July 2021 Archived 2012-12-18 at the Wayback Machine
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
