 | |
| Names | |
|---|---|
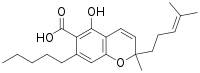
| IUPAC name
5-Hydroxy-2-methyl-2-(4-methylpent-3-enyl)-7-pentylchromene-6-carboxylic acid | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H30O4 | |
| Molar mass | 358.478 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cannabichromenic acid (CBCA) is minor cannabinoid and precursor of cannabichromene.[2]
Biosynthesis
Geranyl pyrophosphate and olivetolic acid combine to produce cannabigerolic acid (CBGA; the sole intermediate for all other phytocannabinoids). The enzyme CBCA synthase can cyclize either cannabigerolic acid or cannabinerolic acid (the Z isomer of CBGA) to form CBCA.[3]
See also
- Cannabielsoin
- Cannabisin F
References
- ↑ "KNApSAcK Metabolite Information - C00053033". www.knapsackfamily.com.
- ↑ Shoyama Y, Fujita T, Yamauchi T, Nishioka I (June 1968). "Cannabichromenic acid, a genuine substance of cannabichromene". Chemical & Pharmaceutical Bulletin. 16 (6): 1157–8. doi:10.1248/cpb.16.1157. PMID 5706836.
- ↑ Morimoto S, Komatsu K, Taura F, Shoyama Y (November 1998). "Purification and characterization of cannabichromenic acid synthase from Cannabis sativa". Phytochemistry. 49 (6): 1525–9. doi:10.1016/s0031-9422(98)00278-7. PMID 9862135.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.