 | |
| Clinical data | |
|---|---|
| Trade names | Nogexan |
| Other names | Carbubarbital |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
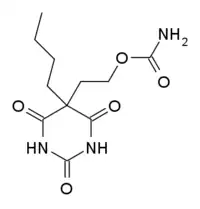
| Formula | C11H17N3O5 |
| Molar mass | 271.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Carbubarb (Carbubarbital, trade name Nogexan) is a carbamate-substituted barbiturate derivative, which has sedative effects.[1]
References
- ↑ US patent 4428887, Tou, J. S. & Schleppnik, A. A., "Method of producing mono-substituted terminal diesters", issued 1984-01-31, assigned to Monsanto
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.