 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C20H20Cl3N4Rh | |
| Molar mass | 525.66 g·mol−1 |
| Appearance | yellow solid |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
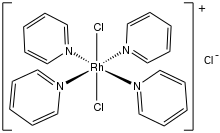
Dichlorotetrakis(pyridine)rhodium(III) chloride is the chloride salt of the coordination complex with the formula [RhCl2(pyridine)4]+. Various hydrates are known, but all are yellow solids. The tetrahydrate initially crystallizes from water.[1] The tetrahydrate converts to the monohydrate upon vacuum drying at 100 °C.
The hydrates of [RhCl2(pyridine)4]Cl are prepared by heating rhodium trichloride with an excess of pyridine in the presence of a catalytic amount of a reductant.[2]
Related complexes
The molecular complex RhCl3(pyridine)3 is an intermediate in the synthesis of [RhCl2(pyridine)4]Cl.[3]
References
- ↑ Vasilchenko, D. B.; Baidina, I. A.; Filatov, E. Yu.; Korenev, S. V. (2009). "Structure and thermal properties of RhPy4Cl2]X Complex Salts (X = Cl−, ReO4−, ClO4−)". Journal of Structural Chemistry. 50 (2): 335–342. doi:10.1007/s10947-009-0046-7.
- ↑ Gillard, R. D.; Wilkinson, G. W. (1967). "trans-Dichlorotetra(pyridine)rhodium(III) Salts". Inorganic Syntheses. 10: 64–67. doi:10.1002/9780470132418.ch11.
- ↑ Acharya, K. R.; Tavale, S. S.; Guru Row, T. N. (1984). "Structure of mer-Trichlorotris(pyridine)rhodium(III), RhCl3(C5H5N)3". Acta Crystallographica Section C Crystal Structure Communications. 40 (8): 1327–1328. doi:10.1107/S0108270184007848.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.