 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.345 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
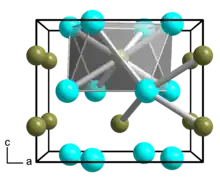
| Ni2B | |
| Molar mass | 128.2 g/mol |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H317, H350i, H372, H410 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dinickel boride is a chemical compound of nickel and boron with formula Ni
2B.[1][2] It is one of the borides of nickel.
The formula "Ni
2B" and the name "nickel boride" are often used for a nickel-boron catalyst obtained by reacting nickel salts with sodium borohydride. However, that product is not a well-defined compound, and its bulk formula is closer to Na
2.5B(sic).[3]
Synthesis
Dinickel boride can be obtained (together with other nickel borides) by heating sodium borohydride with powdered nickel metal up to 670 °C in a closed vessel, so that the released hydrogen creates a pressure of up to 3.4 MPa. The main reactions can be summarized as
- 2NaBH
4 ↔ 2NaH + B
2H
6 - 2Ni + 2B
2H
6 + NaH ↔ Ni
2B + 3BH
3 + 2H
2 + Na
but other reactions occur, yielding other borides.[4]
See also
References
- 1 2 US National Institutes of Health (2020): "Nickel boride (Ni2B)". Compound page at the NCBI PubChem site. Accessed on 2020-07-18.
- ↑ Bjurstrom, T. (March 1933). "Röntgenanalyse der Systeme Eisen-Bor, Kobalt-Bor und Nickel-Bor" [X-ray analysis of the iron-boron, cobalt-boron, and nickel-boron systems]. Arkiv för kemi, mineralogi och geologi. 11A (5).
- ↑ Hofer, L. J. E.; Shultz, J. F.; Panson, R. D.; Anderson, R. B. (December 1964). "The Nature of the Nickel Boride Formed by the Action of Sodium Borohydride on Nickel Salts". Inorganic Chemistry. 3 (12): 1783–1785. doi:10.1021/ic50022a031. ISSN 0020-1669.
- ↑ Shahbazi, Mahboobeh; Cathey, Henrietta; Danilova, Natalia; Mackinnon, Ian (2018-07-22). "Single Step Process for Crystalline Ni-B Compounds". Materials. 11 (7): 1259. Bibcode:2018Mate...11.1259S. doi:10.3390/ma11071259. ISSN 1996-1944. PMC 6073427. PMID 30037149.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.