 | |
| Names | |
|---|---|
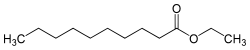
| Preferred IUPAC name
Ethyl decanoate | |
| Other names
Decanoic acid ethyl ester Ethyl caprate Ethyl caprinate Ethyl decylate Capric acid ethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.421 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H24O2 | |
| Molar mass | 200.322 g·mol−1 |
| Density | 0.862 g/cm3 |
| Melting point | −26 °C (−15 °F; 247 K) |
| Boiling point | 245 °C (473 °F; 518 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ethyl decanoate, also known as ethyl caprate, is a fatty acid ester formed from capric acid and ethanol. This ester is a frequent product of fermentation during winemaking, especially at temperatures above 15 °C.[1]
References
- ↑ Killian, E.; Ough, C. S. (1979). "Fermentation Esters — Formation and Retention as Affected by Fermentation Temperature". American Journal of Enology and Viticulture. 30 (4): 301–305.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.