 | |
| Names | |
|---|---|
| Systematic IUPAC name
Germanium(IV) sulfide[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.537 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| GeS2 | |
| Molar mass | 136.75 g·mol−1 |
| Appearance | White, translucent crystals |
| Density | 2.94 g cm−3 |
| Melting point | 840 °C (1,540 °F; 1,110 K) |
| Boiling point | 1,530 °C (2,790 °F; 1,800 K) |
| 0.45 g/100 mL | |
| Solubility | soluble in liquid ammonia |
| −53.3·10−6 cm3/mol | |
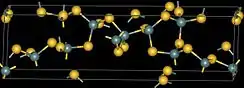
| Structure | |
| monoclinic, mP36 | |
| Pc, No. 7 | |
| tetrahedral at Ge, bent at S | |
| Thermochemistry | |
Heat capacity (C) |
50 J /(mol K) |
Std enthalpy of formation (ΔfH⦵298) |
-150.06 kJ/mol |
| Related compounds | |
Related compounds |
Carbon disulfide Germanium dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Germanium disulfide or Germanium(IV) sulfide is the inorganic compound with the formula GeS2. It is a white high-melting crystalline solid.[1][2] The compound is a 3-dimensional polymer,[3][4] in contrast to silicon disulfide, which is a one-dimensional polymer. The Ge-S distance is 2.19 Å.[3]
Isolation, production, reactions
Germanium disulfide was first found in samples of argyrodite. The fact that germanium sulfide does not dissolve in aqueous acid facilitated its isolation.[5]
Germanium disulfide is produced by treating a solution of germanium tetrachloride in a concentrated hydrochloric acid solution with hydrogen sulfide. It precipitates as a white solid.[6]
It is insoluble in water, it dissolves in aqueous solutions of sodium sulfide owing to the formation of thiogermanates:
- GeS2 + Na2S → Na2GeS3
Natural occurrence
Natural GeS2 is restricted to fumaroles of some burning coal-mining waste heaps.[7]
References
- 1 2 Johnson, O. H. (1952). "Germanium and its Inorganic Compounds". Chemical Reviews. 51 (3): 431–469. doi:10.1021/cr60160a002.
- ↑ Golubkov, A. V.; Dubrovskii, G. B.; Shelykh, A. I. (1998). "Preparation and properties of GeS2 single crystals". Semiconductors. 32 (7): 734–735. Bibcode:1998Semic..32..734G. doi:10.1134/1.1187494. S2CID 101832592.
- 1 2 Zachariasen, W. H. (1936). "The Crystal Structure of Germanium Disulphide". Journal of Chemical Physics. 4 (9): 618–619. Bibcode:1936JChPh...4..618Z. doi:10.1063/1.1749915.
- ↑ Kulikova, L. F.; Lityagina, L. M.; Zibrov, I. P.; Dyuzheva, T. I.; Nikolaev, N. A.; Brazhkin, V. V. (2014). "High-pressure, high-temperature study of GeS2 and GeSe2". Inorg. Mater. 50 (8): 768–774. doi:10.1134/S002016851408010X. S2CID 98354736.
- ↑ Winkler, C. (1886). "Mittheilungen über das Germanium". Journal für Praktische Chemie. 34 (1): 177–229. doi:10.1002/prac.18860340122.
- ↑ P. W. Schenk (1963). "Germanium Disulfide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2pages=723-724. NY,NY: Academic Press.
- ↑ "Unnamed (Ge Sulphide)".