 | |
| Names | |
|---|---|
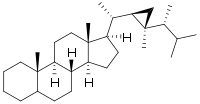
| IUPAC name
5ξ-Gorgostane[1] | |
| Systematic IUPAC name
(3aS,3bR,5Ξ,9aS,9bS,11aR)-9a,11a-Dimethyl-1-[(1S)-1-{(1R,2R)-2-methyl-2-[(2R)-3-methylbutan-2-yl]cyclopropyl}ethyl]hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C30H52 | |
| Molar mass | 412.746 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gorgostane is a steroid triterpene, its derivative distributed in corals, hence the name. Compared with other steroids, there is a cyclopropane ring in the 17C side-chain.[2]
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1530. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Elshamy AI, Abdel-Razik AF, Nassar MI, Mohamed TK, Ibrahim MA, El-Kousy SM (2013). "A new gorgostane derivative from the Egyptian Red Sea soft coral Heteroxenia ghardaqensis". Nat Prod Res. 27 (14): 1250–1254. doi:10.1080/14786419.2012.724417. PMID 22967306. S2CID 2623579.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.