 | |
| Names | |
|---|---|
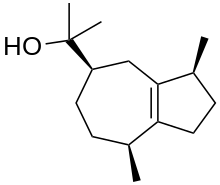
| IUPAC name
Guai-1(5)-en-11-ol | |
| Systematic IUPAC name
2-[(3S,5R,8S)-3,8-Dimethyl-1,2,3,4,5,6,7,8-octahydroazulen-5-yl]propan-2-ol | |
| Other names
Champacol, 5-Azulenemethanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.003 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.372 g·mol−1 |
| Density | 0.961 g/mL |
| Melting point | 92 °C (198 °F; 365 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Guaiol or champacol is an organic compound, a sesquiterpenoid alcohol found in several plants, especially in the oil of guaiacum and cypress pine.[1] It is a crystalline solid that melts at 92 °C.[2] Guaiol is one of many terpenes found in Cannabis and it has been associated with anxiolytic activity.[3][4]
Reactions
Guaiol yields a deep purple color when treated with electrophilic bromine reagents.[5]
See also
References
- ↑ The Merriam-Webster Dictionary.
- ↑ Wolfram Alpha Guaiol
- ↑ Hillig, Karl W (2004-10-01). "A chemotaxonomic analysis of terpenoid variation in Cannabis". Biochemical Systematics and Ecology. 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004.
- ↑ Kamal, Brishna S.; Kamal, Fatima; Lantela, Daniel E. (2018). "Cannabis and the Anxiety of Fragmentation—A Systems Approach for Finding an Anxiolytic Cannabis Chemotype". Frontiers in Neuroscience. 12: 730. doi:10.3389/fnins.2018.00730. PMC 6204402. PMID 30405331.
- ↑ Waddell, TG; Arp, NW; Bodine, KD; Pagni, RM (2002). "The guaiol color reaction". Planta Medica. 68 (10): 949–50. doi:10.1055/s-2002-34931. PMID 12391567.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.