 | |
| Names | |
|---|---|
| Preferred IUPAC name
1H-Inden-1-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H6O | |
| Molar mass | 130.146 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
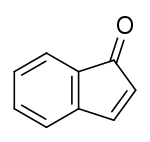
Indenone is a polycyclic ketone with chemical formula C9H6O. It is composed of a benzene ring fused with a cyclopentenone ring. Indenones can be used as intermediates in the synthesis of more complex molecules.[1]
See also
References
- ↑ Larock, R. C.; M. J. Doty; S. Cacchi (1993). "Synthesis of indenones via palladium-catalyzed annulation of internal alkynes". J. Am. Chem. Soc. 58 (17): 4579–4583. doi:10.1021/jo00069a017.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.