 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Amino-3H-pyrimidin-4-one | |
| Other names
2-Aminouracil | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.266 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H5N3O | |
| Molar mass | 111.104 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Isocytosine or 2-aminouracil is a pyrimidine base that is an isomer of cytosine. It is used in combination with isoguanine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.[1] In particular, it is used as a nucleobase of hachimoji RNA.[2]
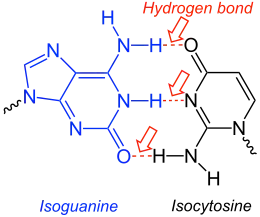
Isoguanine-Isocytosine-base-pair
It can be synthesized from guanidine and malic acid.[3]
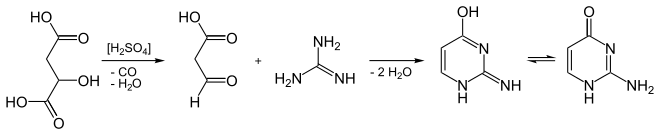
Synthesis of isocytosine from malic acid
It is also used in physical chemical studies involving metal complex binding, hydrogen bonding, and tautomerism and proton transfer effects in nucleobases.[4]
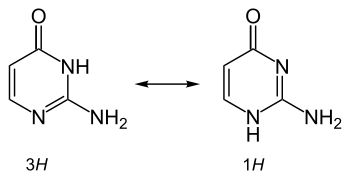
Tautomerism of isocytosine
References
- ↑ "Isocytosine". Molecule of the Week. American Chemical Society. Retrieved November 1, 2012.
- ↑ Hoshika, Shuichi; et al. (22 February 2019). "Hachimoji DNA and RNA: A genetic system with eight building blocks". Science. 363 (6429): 884–887. doi:10.1126/science.aat0971. PMC 6413494. PMID 30792304.
- ↑ William T. Caldwell , Harry B. Kime (1940). "A New Synthesis of Isocytosine". J. Am. Chem. Soc. 62 (9): 2365–2365. doi:10.1021/ja01866a028.
- ↑ "Isocytosine". Sigma-Aldrich. Retrieved November 1, 2012.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.