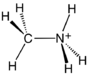
| |||
 Methylammonium bromide crystals | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methylazanium bromide | |||
| Systematic IUPAC name
Methanaminium bromide | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.027.255 | ||
| EC Number |
| ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH3NH3Br | |||
| Molar mass | 111.96904 g/mol | ||
| Appearance | White crystals [1] | ||
| Melting point | 296[2] °C (565 °F; 569 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
irritant | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Methylammonium bromide in an organic halide with a formula of CH3NH3Br. It is the salt of methylammonium and bromide. It is a colorless, water-soluble solid.
The methylammonium halides are precursors to perovskite solar cells, which are being evaluated.[3]
References
- ↑ "Methylammonium bromide". Greatcell Solar Materials. Retrieved 7 May 2021.
- ↑ "Sigma-Aldrich". Sigma-Aldrich. Retrieved 5 February 2017.
- ↑ Li, Hangqian. (2016). "A modified sequential deposition method for fabrication of perovskite solar cells". Solar Energy. 126: 243–251. Bibcode:2016SoEn..126..243L. doi:10.1016/j.solener.2015.12.045.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.