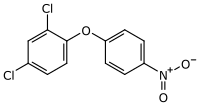
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,4-Dichloro-1-(4-nitrophenoxy)benzene | |
| Other names
Nitrophen; Nitrofene; 2,4-Dichlorophenyl 4-nitrophenyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.824 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C12H7Cl2NO3 | |
| Molar mass | 284.09 g·mol−1 |
| Appearance | Colorless, crystalline solid |
| Density | 1.80 g/cm3 at 83 °C |
| Melting point | 64–71 °C (147–160 °F; 337–344 K) (technical) |
| 0.7-1.2 mg/L at 22 °C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Nitrofen is an herbicide of the diphenyl ether class. Because of concerns about its carcinogenicity, the use of nitrofen has been banned in the European Union[2] and in the United States since 1996.[1][3] It has been superseded by related protoporphyrinogen oxidase enzyme inhibitors including acifluorfen and fomesafen.
In 2002, Nitrofen was detected in organic feed, organic eggs, and organic poultry products in Germany prompting a scandal which caused a decline in all organic meat sales in Europe.[4][5]
Nitrofen is listed as an IARC Group 2B carcinogen, meaning it is "possibly carcinogenic to humans".[6]
References
- 1 2 Nitrofen data sheet, INCHEM WHO/FAO report, March 1999.
- ↑ Banned pesticide in German grain, Pesticides News No. 57, September 2002, page 22
- ↑ Pesticide Properties Database. "Nitrofen". University of Hertfordshire. Retrieved 2021-03-03.
- ↑ Nitrofen scandal causes organic meat sales to dip, Just Food, October 2, 2002.
- ↑ Organic scandal halts Germany's green revolution, by John Hooper, The Guardian, June 12, 2002.
- ↑ IARC Monographs - Classifications - by Group
External links
- Nitrofen in the Pesticide Properties DataBase (PPDB)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.