 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ovalene[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.347 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C32H14 | |
| Molar mass | 398.45 g/mol |
| Density | 1.496 g/cm3[2] |
| Melting point | 473 °C (883 °F; 746 K)[2] |
| -353.8·10−6 cm3/mol[3] | |
| Structure[2] | |
| monoclinic, P21/a | |
a = 1.947(5) nm, b = 0.470(1) nm, c = 1.012(4) nm α = 90°, β = 105.0(3)°, γ = 90° | |
Formula units (Z) |
2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
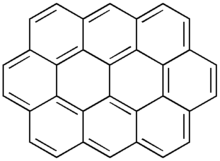
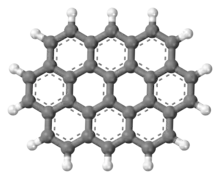
Ovalene is a polycyclic aromatic hydrocarbon with the formula C32H14, which consists of ten peri-fused six-membered rings. It is very similar to coronene.
Ovalene is a reddish-orange compound. It is sparingly soluble in solvents such as benzene, toluene, and dichloromethane. Its solutions have a green fluorescence under UV light.
Ovalene has been shown to form in deep-sea hydrothermal vent areas and in the hydrocracking process of petroleum refining.
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 205. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 Donaldson, D. M.; Robertson, J. M. (1953). "The crystal and molecular structure of ovalene a quantitative X-ray investigation". Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 220 (1141): 157–170. Bibcode:1953RSPSA.220..157D. doi:10.1098/rspa.1953.0179. S2CID 98584903.
- ↑ Akamatu, Hideo; Inokuchi, Hiroo; Handa, Takashi (1951). "Electrical Conductivity and Magnetic Susceptibility of Ovalene". Nature. 168 (4273): 520–521. Bibcode:1951Natur.168..520A. doi:10.1038/168520b0. S2CID 4172683.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.