 | |
 | |
 Coloured water (top) and perfluoroheptane (bottom). Perfluoroheptane is hydrophobic and is denser than water, so it sinks to the bottom and the animals pictured cannot penetrate it. | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexadecafluoroheptane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.812 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
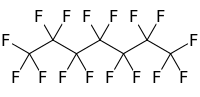
| C7F16 | |
| Molar mass | 388.051 g·mol−1 |
| Appearance | clear liquid[1] |
| Density | 1.706 g/cm3 |
| Boiling point | 80~82°C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Perfluoroheptane, C7F16, (usually referring to the straight chain molecule called n-perfluoroheptane) is a perfluorocarbon.[2] It is hydrophobic (water-insoluble) and oleophobic (oil-insoluble). It is used in deacidification of paper as a medium carrying powdered magnesium oxide.[3]
References
- 1 2 "Perfluoro-n-heptane Safety Data Sheet" (PDF). Exfluor Research Corporation. Retrieved 2020-04-30.
- ↑ Pubchem (USG) page on perfluoroheptane
- ↑ Porck, Henk J. (1996). Mass Deacidification: An Update on Possibilities and Limitations (PDF). Washington D.C.: Commission on Preservation and Access. p. 16. ISBN 1887334521. Retrieved 2015-12-09.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.