 | |
| Names | |
|---|---|
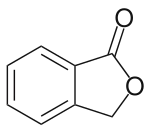
| Preferred IUPAC name
2-Benzofuran-1(3H)-one | |
| Other names
Phthalolactone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.586 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6O2 | |
| Molar mass | 134.134 g·mol−1 |
| Melting point | 75 to 77 °C (167 to 171 °F; 348 to 350 K)[1] |
| Boiling point | 290 °C (554 °F; 563 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phthalide is an organic chemical compound with the molecular formula C8H6O2. It is a white solid and the simplest benzo lactone. It is prepared from hydroxymethylbenzoic acid.[3]
Phthalides
The phthalide core is found a variety of more complex chemical compounds including dyes (such as phenolphthalein), fungicides (such as tetrachlorophthalide, often referred to simply as "phthalide"), and natural oils (such as butylphthalide).
Examples
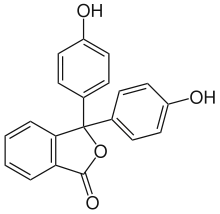 Phenolphthalein
Phenolphthalein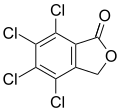 Tetrachlorophthalide
Tetrachlorophthalide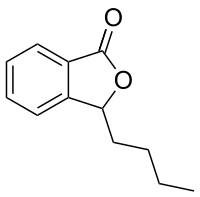
References
- ↑ Kumar, R. Arun; Maheswari, C. Uma; Ghantasala, Satheesh; Jyothi, C.; Reddy, K. Rajender (2011). "Synthesis of 3H-Quinazolin-4-ones and 4H-3,1-Benzoxazin-4-ones via Benzylic Oxidation and Oxidative Dehydrogenation using Potassium Iodide-tert-Butyl Hydroperoxide". Advanced Synthesis & Catalysis. 353 (2+3): 401–410. doi:10.1002/adsc.201000580.
- ↑ Kus, Nermin Simsek (2008). "Some oxidation reactions with molecular oxygen in subcritical water". Asian Journal of Chemistry. 20 (2): 1226–1230.
- ↑ J. H. Gardner, C. A. Naylor, Jr (1936). "Phthalide". Organic Syntheses. 16: 71. doi:10.15227/orgsyn.016.0071.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.