 | |
 | |
| Names | |
|---|---|
| IUPAC name
(Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate | |
| Other names
PyBOP | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.125.168 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H28F6N6OP2 | |
| Molar mass | 520.401 g·mol−1 |
| Appearance | White crystals |
| Melting point | 150 °C (302 °F; 423 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling:[1][2] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
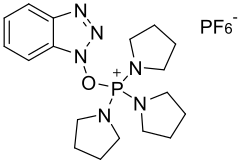
PyBOP (benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate) is a peptide coupling reagent used in solid phase peptide synthesis. It is used as a substitute for the BOP reagent - avoiding the formation of the carcinogenic waste product HMPA.[3]
See also
- BOP reagent
- DEPBT, a related reagent that contains no phosphorus-nitrogen bonds
- HATU
- HBTU
References
- ↑ Sigma-Aldrich Co., product no. {{{id}}}.
- ↑ GHS: Sigma-Aldrich377848
- ↑ Coste, J.; Le-Nguyen, D.; Castro, B. (1990). "PyBOP®: A new peptide coupling reagent devoid of toxic by-product". Tetrahedron Letters. 31 (2): 205. doi:10.1016/S0040-4039(00)94371-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.