 | |
| Names | |
|---|---|
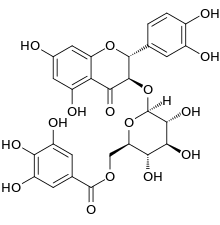
| IUPAC name
(2R,3R)-3′,4′,5,7-Tetrahydroxy-4-oxoflavan-3-yl β-D-glucopyranoside 6-(3,4,5-trihydroxybenzoate) | |
| Systematic IUPAC name
[(2R,3S,4S,5R,6S)-6-{[(2R,3R)-2-(3,4-Dihydroxymethyl)-5,7-dihydroxy-4-oxo-2,3-dihydro-2H-1-benzopyran-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methyl 3,4,5-trihydroxybenzoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C28H26O16 | |
| Molar mass | 618.500 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Taxillusin is a flavonol found in the parasitic plant Taxillus kaempferi.[1][2] It is a galloylated 3-O-glucoside of quercetin.
References
- ↑ Konishi T, Nishio T, Kiyosawa S, Fujiwara Y, Konoshima T (February 1996). "The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Taxillus kaempferi". Yakugaku Zasshi (in Japanese). 116 (2): 148–157. doi:10.1248/yakushi1947.116.2_148. PMID 8717281.
- ↑ Atsushi Sakurai & Yasuaki Okumura (1983). "Chemical studies on the mistletoe. V. The structure of taxillusin, a new flavonoid glycoside isolated from Taxillus kaempferi". Bulletin of the Chemical Society of Japan. 56 (2): 542–544. doi:10.1246/bcsj.56.542.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.