| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
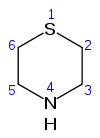
Thiomorpholine[1] | |||
| Other names
Thiamorpholine | |||
| Identifiers | |||
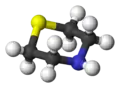
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.238 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H9NS | |||
| Molar mass | 103.18 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Strong odor resembling piperidine[2] | ||
| Boiling point | 169 °C (336 °F; 442 K)[2] | ||
| Miscible[2] | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Thiomorpholine, C4H9NS, is a heterocyclic compound containing nitrogen and sulfur.[3] It can be considered a thio analog of morpholine.
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 142. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- 1 2 3 Merck Index, 12th Edition, monograph 9435, p. 1587
- ↑ "Thiomorpholine". ChemSpider. Retrieved 18 April 2015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.