 | |
| Names | |
|---|---|
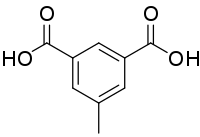
| Preferred IUPAC name
5-Methylbenzene-1,3-dicarboxylic acid | |
| Other names
5-Methyl-1,3-benzenedicarboxylic acid; 3,5-Dicarboxytoluene; 5-Methylisophthalic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.166 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H8O4 | |
| Molar mass | 180.159 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.4±0.1 g/cm3 |
| Melting point | 298 °C |
| Boiling point | 408.7±33.0 °C at 760 mmHg |
| Vapor pressure | 0.0±1.0 mmHg at 25°C |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | 215.1±21.9 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uvitic acid (5-methylisophthalic acid) is an organic compound with the formula CH3C6H3(COOH)2.[1][2] The name comes from Latin uva which means a grape. The acid is called so because it may be produced indirectly from tartaric acid, which is found in the grape.[3] Under normal conditions, the acid is a white crystalline substance.
Preparation
Uvitic acid is obtained by oxidizing mesitylene.[4]
See also
References
- ↑ "Uvitic acid". rdchemicals.com. Retrieved 31 October 2016.
- ↑ "Uvitic acid". chemspider.com. Retrieved 31 October 2016.
- ↑ Senning, Alexander (2006). Elsevier's Dictionary of Chemoetymology: The Whys and Whences of Chemical Nomenclature and Terminology. Elsevier. p. 410. ISBN 9780080488813. Retrieved 31 October 2016.
- ↑ "Definition of uvitic acid". merriam-webster.com. Retrieved 31 October 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.