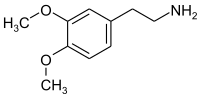
3,4-Dimethoxyphenethylamine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(3,4-Dimethoxyphenyl)ethan-1-amine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.979 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C10H15NO2 |
| Molar mass | 181.23 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3,4-Dimethoxyphenethylamine (DMPEA) is a chemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine.
Chemistry
One of the earliest syntheses of DMPEA (then referred to as "homoveratrylamine") was that of Pictet and Finkelstein, who made it in a multi-step sequence starting from vanillin.[1] A similar sequence was subsequently reported by Buck and Perkin,[2] as follows:
- 3,4-Dimethoxybenzaldehyde (veratraldehyde) → 3,4-Dimethoxycinnamic acid → 3,4-Dimethoxyphenylpropionic acid → 3,4-Dimethoxyphenylpropionamide → 3,4-Dimethoxyphenethylamine
A much shorter synthesis is given by Shulgin and Shulgin:[3][4]
Derivatives
A known use was in the synthesis of Bevantolol.
Pharmacology
DMPEA has some activity as a monoamine oxidase inhibitor.[5]
Occurrence
DMPEA occurs naturally along with mescaline in various species of cacti such as San Pedro and Peruvian Torch.[6][7][8]
See also
- 3-Methoxytyramine
- Mescaline
References
- ↑ A. Pictet and M. Finkelstein (1909). "Synthese des Laudanosins." Ber. 42 1979-1989.
- ↑ J. S. Buck and W. H. Perkin (1924). "CCXVIII. Ψ-epiBerberine." J. Chem. Soc., Trans. 125 1675-1686.
- ↑ A. Shulgin and A. Shulgin (1991). "PiHKAL A Chemical Love Story", pp. 614-616, Transform Press, Berkeley. ISBN 0-9630096-0-5
- ↑ "Erowid Online Books : "PIHKAL" - #60 DMPEA".
- ↑ Keller WJ; Ferguson GG (July 1977). "Effects of 3,4-dimethoxyphenethylamine derivatives on monoamine oxidase". Journal of Pharmaceutical Sciences. 66 (7): 1048–50. doi:10.1002/jps.2600660741. PMID 886445.
- ↑ Lundström J (December 1970). "Biosynthesis of mescaline and 3,4-dimethoxyphenethylamine in Trichocereus pachanoi Br&R". Acta Pharmaceutica Suecica. 7 (6): 651–66. PMID 5511715.
- ↑ Pummangura S; Nichols DE; McLaughlin JL (October 1977). "Cactus alkaloids XXXIII: beta-phenethylamines from the Guatemalan cactus Pilosocereus maxonii". Journal of Pharmaceutical Sciences. 66 (10): 1485–7. doi:10.1002/jps.2600661037. PMID 925910.
- ↑ Pardanani JH; McLaughlin JL; Kondrat RW; Cooks RG (1977). "Cactus alkaloids. XXXVI. Mescaline and related compounds from Trichocereus peruvianus". Lloydia. 40 (6): 585–90. PMID 600028.