ALX-1393
 | |
| Names | |
|---|---|
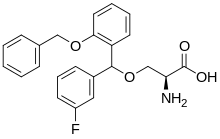
| IUPAC name
(2S)-2-Amino-3-[(3-fluorophenyl)-(2-phenylmethoxyphenyl)methoxy]propanoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H22FNO4 |
| Molar mass | 395.430 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
ALX-1393 is a glycine reuptake inhibitor.
Pharmacodynamics
ALX-1393 works by inhibiting the action of GLYT2.[2] This causes elevated levels of glycine, an inhibitory neurotransmitter.
Potential uses
ALX-1393 has been shown to have potential as an analgesic. This is thought to be due to the elevated glycine levels reducing the transmission of the pain signals.[3]
Tests have shown that it was able to help reduce cancer pain in a potent way.[4]
References
- ↑ "(2S)-2-amino-3-{[2-(benzyloxy)phenyl](3-fluorophenyl)methoxy}propanoic acid".
- ↑ Eckle, V.-S.; Antkowiak, B. (2013-12-03). "ALX 1393 inhibits spontaneous network activity by inducing glycinergic tonic currents in the spinal ventral horn". Neuroscience. 253: 165–171. doi:10.1016/j.neuroscience.2013.08.042. ISSN 1873-7544. PMID 23994185. S2CID 30039776.
- ↑ Benito-Muñoz, Cristina; Perona, Almudena; Felipe, Raquel; Pérez-Siles, Gonzalo; Núñez, Enrique; Aragón, Carmen; López-Corcuera, Beatriz (2021-06-02). "Structural Determinants of the Neuronal Glycine Transporter 2 for the Selective Inhibitors ALX1393 and ORG25543". ACS Chemical Neuroscience. 12 (11): 1860–1872. doi:10.1021/acschemneuro.0c00602. ISSN 1948-7193. PMC 8691691. PMID 34003005.
- ↑ Motoyama, Naoyo; Morita, Katsuya; Shiraishi, Seiji; Kitayama, Tomoya; Kanematsu, Takashi; Uezono, Yasuhito; Dohi, Toshihiro (October 2014). "Relief of cancer pain by glycine transporter inhibitors". Anesthesia and Analgesia. 119 (4): 988–995. doi:10.1213/ANE.0000000000000388. ISSN 1526-7598. PMID 25076101.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.