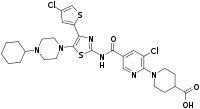
Avatrombopag
 | |
| Names | |
|---|---|
| Pronunciation | a" va trom' boe pag |
| Trade names | Doptelet |
| Other names | Avatrombopag maleate |
IUPAC name
| |
| Clinical data | |
| Drug class | Thrombopoietin receptor agonist[1] |
| Main uses | Low platelets in chronic liver disease[2][3] |
| Side effects | Fever, abdominal pain, nausea, headache, tiredness, peripheral swelling[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 40 to 60 mg OD[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618032 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C29H34Cl2N6O3S2 |
| Molar mass | 649.65 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Avatrombopag, sold under the brand name Doptelet, is a medication used to treat low platelets in chronic liver disease when an invasive medical procedure is required.[2][3] It is taken by mouth.[1]
Common side effects include fever, abdominal pain, nausea, headache, tiredness, and peripheral swelling.[2] Other side effects may include blood clots.[2] Use in pregnancy may harm the baby.[2] Safety in pregnancy or breastfeeding is unclear.[1] It is a thrombopoietin receptor agonist, which increases platelet production.[1]
Avatrombopag was approved for medical use in the United States in 2018 and Europe in 2019.[2][3] In the United Kingdom a course of treatment costs the NHS £640 to £960 as of 2020.[4] In the United States this amount is 3,650 USD to 5,500 USD.[5]
Medical uses
Dosage
It should be started 10 days before the procedure.[3] It is used at a dose of 40 to 60 mg per day, depending on a persons platelets, for 5 days.[2]
References
- 1 2 3 4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1079. ISBN 978-0857114105.
- 1 2 3 4 5 6 7 8 "Avatrombopag Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 16 January 2022.
- 1 2 3 4 5 "Doptelet EPAR". European Medicines Agency (EMA). 24 April 2019. Archived from the original on 22 May 2020. Retrieved 2 May 2020.
- ↑ "Avatrombopag for treating thrombocytopenia in people with chronic liver disease needing a planned invasive procedure". Archived from the original on 17 January 2022. Retrieved 16 January 2022.
- ↑ "Doptelet Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 April 2021. Retrieved 16 January 2022.
External links
| External sites: |
|
|---|---|
| Identifiers: |