 | |
| Names | |
|---|---|
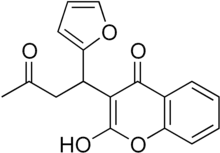
| IUPAC name
3-[1-(2-furyl)-3-oxobutyl]-2-hydroxy-4-chromenone | |
| Other names
Coumafuryl, Ratafin, Fumarine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.814 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C17H14O5 | |
| Molar mass | 298.29 g/mol |
| Density | 1.36 g/cm3 |
| Melting point | 124 |
| 538 mg/L [20 °C] | |
| log P | 1.6 |
| Hazards | |
| Flash point | 214.2 °C (417.6 °F; 487.3 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Fumarin, also known as coumafuryl is a coumarin derivative, a structural analog of warfarin. It can be used as rodenticide.[2][3][4]
References
- ↑ Pesticide Properties Database. "Coumafuryl". University of Hertfordshire.
- ↑ Sato, Shouichi (2005). "Coumarin rodenticides". Drugs and Poisons in Humans. pp. 599–608. doi:10.1007/3-540-27579-7_66. ISBN 3540222774.
- ↑ Jin, Mi‐Cong; Xu, Guo‐Zhang; Ren, Yi‐Ping; Chen, Xiao‐Hong; Xu, Xiao‐Ming (2008). "Identification and determination of coumateralyl and coumafuryl in animal tissues by high‐performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry". Journal of Applied Toxicology. 28 (5): 621–627. doi:10.1002/jat.1313. PMID 17975848. S2CID 25178142.
- ↑ "Compendium of Pesticide Common Names". BCPC.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.