 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
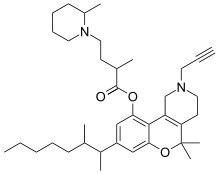
| Formula | C37H56N2O3 |
| Molar mass | 576.866 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Menabitan (INN; SP-204), or menabitan hydrochloride (USAN), is a synthetic drug which acts as a potent cannabinoid receptor agonist.[1][2] It is closely related to natural cannabinoids of the tetrahydrocannabinol (THC) group, differing mainly by its longer and branched side chain, and the replacement of the 9-position carbon with a nitrogen.[1] It was studied as an analgesic in the 1970s and was found to possess antinociceptive effects in both humans and animals but was never marketed.[1][3][4]
Due to its structural similarity to the Schedule I/III drug THC it can be treated as a Schedule I drug within the United States legal system under the Federal Analogue Act.
See also
- A-40174 (SP-1)
- A-41988
- Dimethylheptylpyran
- Nabitan
References
- 1 2 3 Green K, Kim K (February 1977). "Acute dose response of intraocular pressure to topical and oral cannabinoids". Proceedings of the Society for Experimental Biology and Medicine. 154 (2): 228–31. doi:10.3181/00379727-154-39643. PMID 402656. S2CID 32785623.
- ↑ Triggle DJ (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. p. 1271. ISBN 978-0-412-46630-4.
- ↑ Reggio PH (1987). "Molecular determinants for cannabinoid activity: refinement of a molecular reactivity template". NIDA Research Monograph. 79: 82–95. PMID 2830539.
- ↑ Gabriel G. Nahas (5 April 1999). Marihuana and Medicine. Humana Press. p. 46. ISBN 978-0-89603-593-5. Retrieved 9 May 2012.
| Phytocannabinoids (comparison) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthetic cannabinoid receptor agonists / neocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric CBRTooltip Cannabinoid receptor ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Endocannabinoid enhancers (inactivation inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Anticannabinoids (antagonists/inverse agonists/antibodies) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.