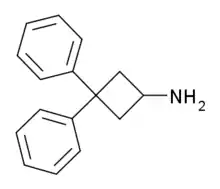
3,3-Diphenylcyclobutanamine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H17N |
| Molar mass | 223.319 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
3,3,-Diphenylcyclobutanamine is a psychostimulant drug which was originally prepared as an antidepressant in the late 1970s.[1] It appears to inhibit the reuptake of serotonin, norepinephrine, and dopamine, and may also induce their release as well.[1] The N-methyl and N,N-dimethyl analogues of the compound are also known and are more potent.[1] All three agents produce locomotor stimulation in animal studies, with the tertiary amine being the strongest.[1]
Synthesis
A number of methods were tried in order to construct the strained four-carbon ring. A synthesis of 3,3-diphenylcyclobutanone appeared in the literature.[2] The ketone was prepared in low yield by the reaction of diphenylketene with 2 equiv of diazomethane.[3] The latter synthesis, although low yielding, was used and the desired amines were prepared from 3,3-diphenylcyclobutanone.
Diphenylketene
Diphenylketene is produced by the elimination of hydrogen chloride from diphenylacetyl chloride in the presence of triethylamine.[4]
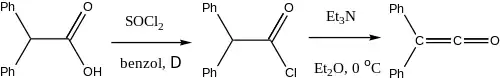 Preparation of Diphenylketene
Preparation of Diphenylketene
See also
- β-Phenylmethamphetamine
- Fezolamine
- Azetidine ring variation:[5]
References
- 1 2 3 4 Carnmalm B, Rämsby S, Renyi AL, Ross SB, Ogren SO (January 1978). "Antidepressant agents. 9. 3,3-Diphenylcyclobutylamines, a new class of central stimulants". Journal of Medicinal Chemistry. 21 (1): 78–82. doi:10.1021/jm00199a014. PMID 22757.
- ↑ Wilt JW, Dabek RA, Welzel KC (1972). "Transannular neophyl rearrangement". The Journal of Organic Chemistry. 37 (3): 425–430. doi:10.1021/jo00968a022.
- ↑ Michejda CJ, Comnick RW (1975). "Acetolysis of 3,3-disubstituted cyclobutyl tosylates". The Journal of Organic Chemistry. 40 (8): 1046–1050. doi:10.1021/jo00896a010.
- ↑ Taylor EC, McKillop A, Hawks GH (1972). Michejda CJ, von Riesen DD, Comnick RW, Baumgarten HE (eds.). "DIPHENYLKETENE [Ethenone, diphenyl-]". Organic Syntheses. 52: 36. doi:10.15227/orgsyn.052.0036.
- ↑ Das M, Weissenfluh A, Ly N, Trudell ML (June 2020). "Synthesis of Simple 3,3-Diarylazetidines from N-Boc-3-arylazetidinols Using Friedel-Crafts Arylation Conditions". The Journal of Organic Chemistry. 85 (12): 8209–8213. doi:10.1021/acs.joc.0c00454. PMID 32449343. S2CID 218873364.