Tinabinol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C23H34O2S |
| Molar mass | 374.58 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
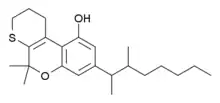
Tinabinol (INN; SP-119) is a synthetic cannabinoid drug and analogue of dronabinol and dimethylheptylpyran which was patented as an antihypertensive but was never marketed.[1][2]
See also
References
- ↑ Brown DT (19 November 1998). Cannabis: The Genus Cannabis. CRC Press. p. 80. ISBN 978-90-5702-291-3. Retrieved 27 April 2012.
- ↑ Negwer M (1994). Organic-chemical drugs and their synonyms: an international survey. Indices. Akad.-Verl. p. 2242. ISBN 978-3-05-501629-5. Retrieved 27 April 2012.
| Phytocannabinoids (comparison) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthetic cannabinoid receptor agonists / neocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric CBRTooltip Cannabinoid receptor ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Endocannabinoid enhancers (inactivation inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Anticannabinoids (antagonists/inverse agonists/antibodies) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.