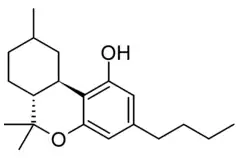
Hexahydrocannabutol
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| Chemical and physical data | |
| Formula | C20H30O2 |
| Molar mass | 302.458 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Hexahydrocannabutol (HHCB, HHC-B) is a semi-synthetic cannabinoid derivative, the hydrogenated derivative of tetrahydrocannabutol (THCB). It was first synthesised by Roger Adams in 1942 and produces only weak cannabinoid-like effects in animals.[1] More recently it has been sold as an ingredient in grey-market cannabinoid products.[2]
See also
References
- ↑ Adams R, Loewe S, Smith CM, McPhee WD (March 1942). "Tetrahydrocannabinol homologs and analogs with marihuana activity. XIII". Journal of the American Chemical Society. 64 (3): 694–697. doi:10.1021/ja01255a061.
- ↑ "Classement des cannabinoïdes par puissance - la verte feuille" [Ranking of cannabinoids by potency - the green leaf]. la-verte-feuille.fr (in French). Archived from the original on 31 March 2023.
| Phytocannabinoids (comparison) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthetic cannabinoid receptor agonists / neocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric CBRTooltip Cannabinoid receptor ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Endocannabinoid enhancers (inactivation inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Anticannabinoids (antagonists/inverse agonists/antibodies) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.