Mepyramine
 | |
| Names | |
|---|---|
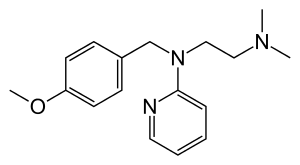
| Other names | Pyrilamine; N-[2-(dimethylamino)ethyl]-N-[(4-methoxyphenyl)methyl]pyridin-2-amine |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | oral, topical, |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a606008 |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C17H23N3O |
| Molar mass | 285.391 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mepyramine, also known as pyrilamine, is a first generation antihistamine, targeting the H1 receptor as an inverse agonist.[1] Mepyramine rapidly permeates the brain, often causing drowsiness.[2] It is often sold as a maleate salt, pyrilamine maleate.
The medication has negligible anticholinergic activity, with 130,000-fold selectivity for the histamine H1 receptor over the muscarinic acetylcholine receptors (for comparison, diphenhydramine had 20-fold selectivity for the H1 receptor).[3]
Medical use
It is used in over-the-counter combination products to treat the common cold and menstrual symptoms such as Midol Complete.[4] It is also the active ingredient of the topical antihistamine creams Anthisan[5] and Neoantergan[1] sold for the treatment of insect bites, stings, and nettle rash.
History
It was patented in 1943 and came into medical use in 1949.[6] It was marketed under the names Histadyl, Histalon, Neo-Antergan, Neo-Pyramine, and Nisaval.[7] In the 1960s and 70s it was a very common component in over-the-counter sleep aids such as "Alva-Tranquil", "Dormin", "Sedacaps", "Sominex", "Nytol", and many others.[7]

See also
- Chloropyramine (chloro instead of methoxy)
References
- 1 2 Parsons ME, Ganellin CR (January 2006). "Histamine and its receptors". British Journal of Pharmacology. 147 (Suppl 1): S127–S135. doi:10.1038/sj.bjp.0706440. PMC 1760721. PMID 16402096.
- ↑ "Mepyramine". drugbank.com. Archived from the original on 16 May 2021. Retrieved 8 May 2021.
- ↑ Kubo N, Shirakawa O, Kuno T, Tanaka C (March 1987). "Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay". Japanese Journal of Pharmacology. 43 (3): 277–282. doi:10.1254/jjp.43.277. PMID 2884340.
- ↑ "Active Ingredients for Midol Complete". Bayer HealthCare LLC. Archived from the original on 2009-12-02. Retrieved 2009-12-08.
- ↑ "Anthisan Cream - Patient Information Leaflet (PIL)". SANOFI Consumer Healthcare. Archived from the original on 2022-09-30. Retrieved 2023-04-02.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 545. ISBN 9783527607495. Archived from the original on 2023-01-12. Retrieved 2023-04-02.
- 1 2 Thornton WE (September 1977). "Sleep aids and sedatives". Jacep. 6 (9): 408–412. doi:10.1016/S0361-1124(77)80006-3. PMID 330911.
External links
| Identifiers: |
|---|
| Antihistamines for topical use | |
|---|---|
| Anesthetics for topical use | |
| Others |
|
| Benzimidazoles (*) | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||