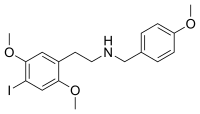
25I-NB4OMe
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | C18H22INO3 |
| Molar mass | 427.282 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
25I-NB4OMe (2C-I-NB4OMe, NB4OMe-2C-I) is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a partial agonist for the human 5-HT2A receptor.[1]
Legality
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[2]
References
- ↑ Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–249. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- ↑ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Statutory Instruments 2014 No. 1106. www.legislation.gov.uk.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.