Nemonapride
 | |
| Clinical data | |
|---|---|
| Trade names | Emilace (JP) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
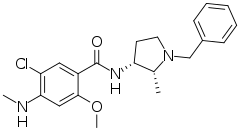
| Formula | C21H26ClN3O2 |
| Molar mass | 387.91 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Nemonapride (エミレース, Emilace (JP)) is an atypical antipsychotic approved in Japan for the treatment of schizophrenia. It was launched by Yamanouchi in May 1991.[1] Nemonapride acts as a D2 and D3 receptor antagonist, and is also a potent 5-HT1A receptor agonist.[2] It has affinity for sigma receptors.
See also
- Benzamide
References
- ↑ "Pharmaceuticals and Medical Devices Safety Information No. 265" (PDF). Pharmaceuticals and Medical Devices Agency. January 2010. Archived from the original (PDF) on 26 July 2022. Retrieved 26 July 2022.
- ↑ Kusumi I, Boku S, Takahashi Y (May 2015). "Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis". Psychiatry and Clinical Neurosciences. 69 (5). Wiley: 243–258. doi:10.1111/pcn.12242. PMID 25296946. S2CID 23102204.
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.