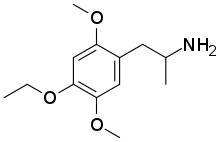
2,5-Dimethoxy-4-ethoxyamphetamine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H21NO3 |
| Molar mass | 239.315 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
2,5-Dimethoxy-4-ethoxyamphetamine (MEM) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin.[1] In his book PiHKAL, he lists the active dose range as 20–50 mg, and the duration as 10–14 hours.[1] According to Shulgin, MEM produces color enhancement, visual phenomena, and pattern movement, among other effects.[1]
MEM possesses affinity (Ki) for the 5-HT2A (3,948 nM), 5-HT2B (64.5 nM), 5-HT7 (7,156 nM), and σ1 (5,077 nM) receptors. It behaves as a partial agonist at the 5-HT2A receptor.[2] MEM is relatively selective for these sites and displays low/negligible (> 10,000 nM) affinity for a wide array of other targets.[2]
See also
- 2,5-Dimethoxy-4-substituted amphetamines
References
- 1 2 3 Shulgin A, Shulgin A (1991). Pihkal: A Chemical Love Story. Transform Press. ISBN 0-9630096-0-5.
- 1 2 Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
External links
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
Sigma receptor modulators | |
|---|---|
| σ1 |
|
| σ2 |
|
| Unsorted |
|
See also: Receptor/signaling modulators | |
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.