CS-27349
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
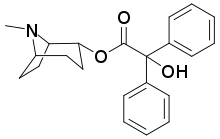
| Formula | C22H25NO3 |
| Molar mass | 351.446 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
CS-27349, or L-2-α-tropinyl benzilate, is an experimental incapacitating agent. It acts as an antagonist to muscarinic acetylcholine receptors, causing delirium. It has 37% of the potency of the related compound 3-quinuclidinyl benzilate (BZ) in producing peripheral effects, but 85% of the potency in producing central effects. The mean dose required to incapacitate subjects was 1.2 times that of BZ.[1] It has not been in use since the 1970s, and there have been no publications about its effects or long-term toxicology since then.[2]
References
- ↑ Ball JC (2015). "Dual Use Research of Concern: Derivatives of 3-Quinuclidinyl Benzilate". Military Medical Science Letters. 84 (1): 2–41. doi:10.31482/mmsl.2015.001.
- ↑ Assessment of Potential Long-Term Health Effects on Army Human Test Subjects of Relevant Biological and Chemical Agents, Drugs, Medications and Substances (PDF) (Report). Ho-Chunk Technical Solutions Healthcare Division. February 29, 2016. Archived (PDF) from the original on December 25, 2020.
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood agents |
| ||||||||||||||
| Blister agents |
| ||||||||||||||
| Nerve agents |
| ||||||||||||||
| Neurotoxins |
| ||||||||||||||
| Pulmonary/ choking agents |
| ||||||||||||||
| Vomiting agents |
| ||||||||||||||
| Incapacitating agents |
| ||||||||||||||
| Lachrymatory agents |
| ||||||||||||||
| Malodorant agents |
| ||||||||||||||
| Cornea-clouding agents |
| ||||||||||||||
| Biological toxins |
| ||||||||||||||
| Other |
| ||||||||||||||
| |||||||||||||||
| mAChRsTooltip Muscarinic acetylcholine receptors |
| ||||
|---|---|---|---|---|---|
| Precursors (and prodrugs) |
| ||||
| |||||
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.