Tretoquinol
 | |
| Names | |
|---|---|
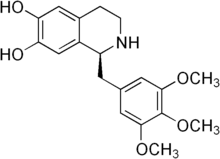
| Preferred IUPAC name
(1S)-1-[(3,4,5-Trimethoxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Tretoquinol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H23NO5 |
| Molar mass | 345.39 g/mol |
| Pharmacology | |
| R03AC09 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tretoquinol is a beta-adrenergic agonist.[1][2]
References
- ↑ Yamato, E.; Hirakura, M.; Sugasawa, S. (1966). "Synthesis of 6,7-dihydrox-1,2,3,4-tetrahydroisoquinoline derivatives". Tetrahedron. 22: 129–134. doi:10.1016/S0040-4020(01)82177-3.
- ↑ Konkar, A. A.; Vansal, S. S.; Shams, G.; Fraundorfer, P. F.; Zheng, W. P.; Nikulin, V. I.; De Los Angeles, J.; Fertel, R. H.; Miller, D. D.; Feller, D. R. (1999-11-01). "β-Adrenoceptor Subtype Activities of Trimetoquinol Derivatives: Biochemical Studies on Human β-Adrenoceptors Expressed in Chinese Hamster Ovary Cells". The Journal of Pharmacology and Experimental Therapeutics. 291 (2). Jpet.aspetjournals.org: 875–883. PMID 10525112. Retrieved 2012-08-20.
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.